TGF-beta and RAS signaling are both required for lung cancer metastasis
When it comes to cancer metastasis, it takes two to tango. That was one of the key findings of a new study: The TGF-beta and RAS signaling pathways work together to spur the spread of cancer in lung adenocarcinoma, a leading cause of cancer deaths around the world.
Take away one of those two signals, and lung cancer will not be able to spread (metastasize) to new parts of the body, their findings in animal models suggest.
The research, published in Cell, points to new opportunities to potentially prevent metastasis, thanks to an updated understanding of the underlying processes.
“About 9 in 10 deaths from cancer are caused by metastasis,” says the study’s first author. “So research to understand, prevent, and treat metastasis has great potential to improve the lives of many people.”
On its own, TGF-beta — a type of signaling protein known as a cytokine — is not an ideal drug target. That’s because it plays a variety of important roles throughout the body, making it nearly impossible to block without risking major side effects.
TGF-beta normally plays critical roles in programs regulating embryonic development and injury repair. But these programs also get activated in cancer cells during metastasis, giving otherwise immobile cells the ability to travel and to invade tissues.
Metastasis in this case, however, also requires inputs from the RAS pathway, the researchers found.
RAS’s normal role is to transmit signals from outside of a cell to the cell nucleus, where it activates genes that control cell growth, division, and differentiation.
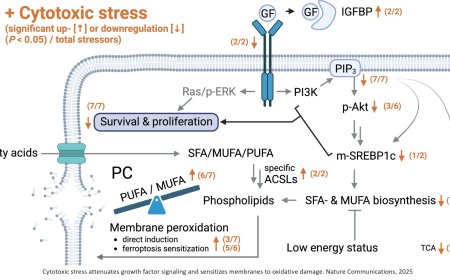
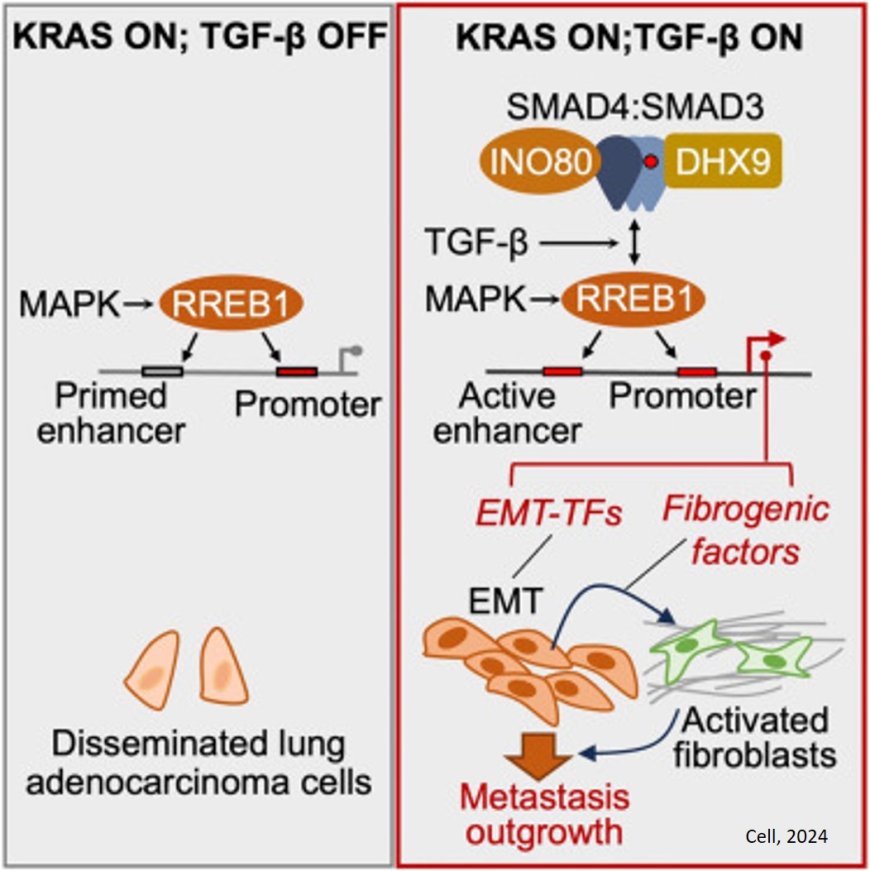
Here, the researchers determined that a transcription factor (a protein that specifically regulates the activity of certain genes) that is controlled by RAS plays a critical role in metastasis. It’s known as RAS response element binding protein 1 (RREB1). They found that RREB1 collaborates with a signaling complex called SMAD4, which is controlled by TGF-beta. And inhibiting RREB1 disabled the metastatic process in mouse models, suggesting it could be a potential new drug target — both in lung cancer and in related diseases like lung fibrosis.
The TGF-beta family of proteins plays a key role in the proliferation and differentiation of many different cell types, controlling the formation and regeneration of tissues from embryonic life to adulthood.
“We know that the TGF-beta pathway promotes injury repair and suppresses the formation of tumors in healthy tissues, but when tumors manage to grow, it promotes metastasis,” the senior author says. “So this is a big question we wanted to understand: How is it that metastasis can take something that is normally present to keep tissues healthy, turn it around, and use it to promote metastatic growth? And this has led to a whole program of research in my lab.”
Another way of putting it is that TGF-beta helps normal cells to regenerate, but when co-opted by cancer, this regeneration turns into runaway growth instead of healthy renewal.
In this study, the team shed new light on the mechanisms at work in the process, revealing that both RAS and TGF-beta inputs are needed for metastasis to take off — at least in this most common form of lung cancer, where the growth of fibrous scar tissue is a key component.
Beyond cancer, TGF-beta-driven lung fibrosis affects hundreds of thousands of people around the world. While targeting TGF-beta has been seen as promising, using TGF-beta inhibitors long-term to treat metastatic cancer or fibrosis might lead to significant side effects. For example, disrupting TGF-beta’s work can lead to excessive activity in the immune system, the author notes.
“So the biotech industry has been looking for ways to target TGF-beta only part-time or in restricted ways,” the author says. “Here we’ve discovered a new candidate for that type of intervention, only focused on a partner signal that we now know is also required for metastasis and fibrosis.”