TMEM65 regulates mitochondrial calcium efflux
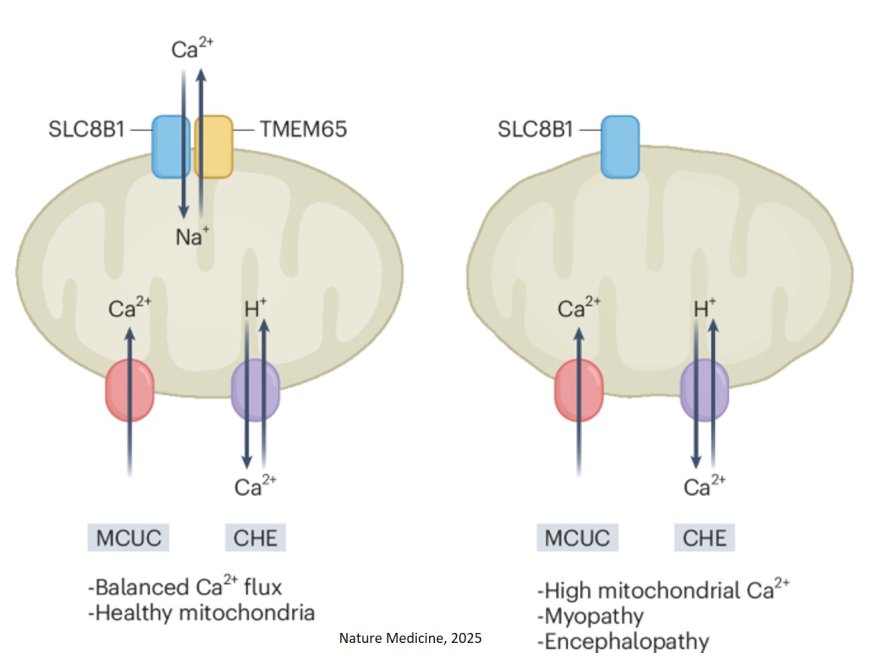
Calcium transport into and out of mitochondria – the powerhouses of cells – is central to cellular energy production and cell death. To maintain the balance of calcium within these powerhouses, cells rely on a protein known as the mitochondrial sodium-calcium exchanger, or NCLX. Now, in new research, scientists have discovered a novel regulator of NCLX activity, a protein called TMEM65, which helps move calcium out of mitochondria, protecting against harmful calcium overload.
The discovery, published in the journal Nature Metabolism, is the first to characterize the interaction of TMEM65 with NCLX in mitochondria. “TMEM65 is the first protein identified that is a bona fide interactor and regulator of NCLX,” explained the senior investigator on the new study. The discovery could help scientists design new therapeutic agents to combat calcium overload of mitochondria in conditions such as heart failure and Alzheimer’s disease.
Mitochondrial calcium exchange serves a critical part in regulating cell survival and pro-energetic signaling pathways. When mitochondria take in too much calcium, which can happen in certain disease states, energy metabolism is disrupted and cells die. This is most apparent in the heart, where calcium overload contributes to the permanent loss of heart muscle cells during heart attacks and in heart failure. It also can result in the loss of brain cells in Alzheimer’s disease and other neurodegenerative conditions.
The authors identified NCLX as a key player in the removal of calcium from mitochondria in the heart and brain. Research has also shown that augmenting NCLX activity can limit the progression of not only heart failure and Alzheimer’s disease but also cancer. Nonetheless, despite these promising findings, an understanding of the mechanisms underlying NCLX regulation has remained elusive.
“NCLX has a very complex structure, which has impeded the study of its regulation and hindered progress in therapeutic development,” the author said. “For our latest study, we decided to take a different approach, using biotin tagging, which allowed us to trace NCLX’s interactions with other proteins in intact cells.”
The team generated a fusion of NCLX and a biotinylation protein. The fusion protein was then placed back into cells, and other proteins that came within its proximity were biotinylated, or biochemically labeled. The biotinylated molecules were then easily isolated, enabling their identification with mass spectrometry. In this way, the researchers ultimately discovered TMEM65 as a primary suspect in NCLX regulation.
“TMEM65 was of particular interest because it is a mitochondrial protein of unknown function,” the author explained. “We also knew about a case report in which a young girl with a loss-of-function mutation in TMEM65 experienced profound muscle weakness and microcephaly (abnormally small head/brain) and neurological dysfunction.”
In subsequent experiments, it was discovered that when TMEM65 is removed from cells, calcium levels in the mitochondria accumulate. This led to the realization that TMEM65 is required for NCLX activity. Its role in regulating NCLX was confirmed in a mouse model in which TMEM65 levels were significantly decreased. As animals matured, they experienced a progressive loss of neuromuscular function, to the extent that they could barely walk by adulthood.
The methods used to identify TMEM65 and to elucidate NCLX regulation are groundbreaking in the field of basic cardiovascular science.
The work has also inspired ongoing investigation of TMEM65. The authors plan next to explore the possibility of modulating TMEM65 activity as a therapeutic strategy. “TMEM65 is a promising therapeutic target,” the author added. “Figuring out how to augment or otherwise alter its interaction with NCLX could offer an important treatment option for patients affected by diseases involving pathogenic calcium buildup in mitochondria.”