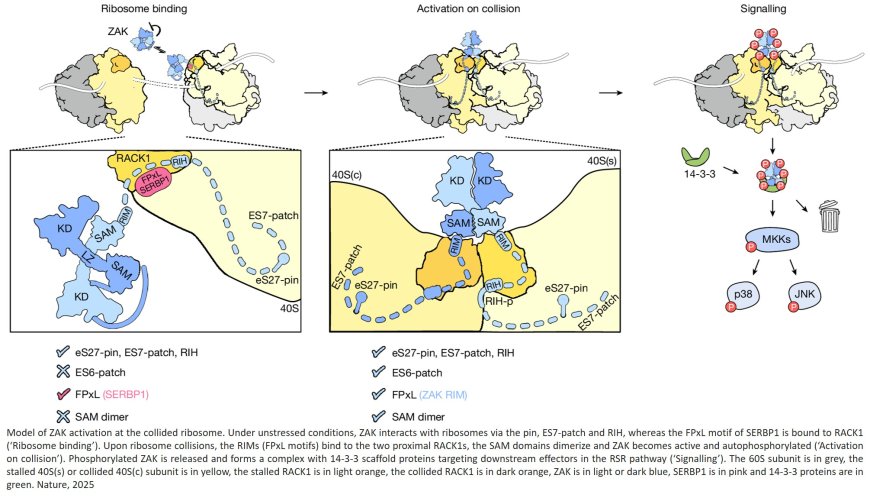
The kinase activity of ZAK at the collided ribosome interface
In an effort to reveal the inner workings of a protein that serves as a cell’s damage detection system, scientists have published what is believed to be the first 3D details of the ZAK protein’s structure.
ZAK has long been known to play a role in regulating cellular stress responses to agents such as UV irradiation. The structure, scientists say, depicts molecular detail down to the atomic level of about a third of the protein, uncovering the mechanism of activation of this protein and setting scientists on a path to eventually develop specialized treatments that target this signaling network.
“In order to develop drugs to target these proteins, we need to understand how they work and how the proteins interact with other parts of the cell,” says the senior author.
The findings, published in Nature are the result of more than two years of work from scientists to determine the shape, structure and action of the ZAK protein.
When a cell is damaged from UV light or is exposed to various stresses including nutrient depletion or various toxins, actively translating ribosomes can stall, disrupting travel along the mRNA, causing collisions and stopping travel. The ribosome traffic jam causes cells to produce incomplete or abnormal proteins and activates the ribotoxic stress response pathway, mediated by ZAK.
“The way the cell knows there's a problem is through ribosomes,” says the senior author, whose team found in 2020 that ribosome collisions activate the ZAK protein.
The new experiments were undertaken to better understand how ZAK proteins “interact directly with ribosomes to sense when something is wrong with protein translation and communicate this to downstream signaling factors,” says the first author of the current research.
For the new studies, the team used lab-cultured human cells to study ZAK signaling dynamics upon ribosome collisions. Typically, ZAK proteins are not abundant in these cells, so the authors engineered them to overproduce inactivated ZAK proteins. Then, they used a drug that causes ribosomes to pause on the mRNA during translation to induce ribosome collisions and activate ZAK. The ZAK protein had a molecular tag that enabled the scientists to grab an enriched sample of activated ZAK protein bound to ribosomes.
With the samples containing activated ZAK proteins, they used cryo-electron microscopy (cryo-EM) to visualize the protein and to define a 3D structure.
Looking at the protein sequence and predicted structural elements, more than half of ZAK is predicted to be unstructured, “like spaghetti,” says the senior author. The other portions looked more structured, the scientists say. The protein may float around the cell with the “spaghetti” end acting as a tentacle to hang onto and ultimately detect collided ribosomes. The two halves of ZAK appear to form a bridge that connects two collided ribosomes, they say.
The research revealed multiple specific interactions of the ZAK protein with the ribosome. They found that the so-called C terminus, or end, of ZAK binds to a ribosome regardless of its collision state, and makes collision-specific interactions with ribosomal rRNA known as expansion segments. They further found that an area near the middle of the ZAK protein, called the RIM, interacts with RACK1 on the ribosome to activate ZAK when ribosomes collide.
The senior author says the new findings about ZAK’s inner workings may advance insights into other kinase proteins like ZAK. Kinases are proteins that add chemical groups to other proteins, acting like on-off switches. Most drugs that target kinases bind to a location that tends to induce side effects. “Now, we know more about the makeup of these specialized sites in the ZAK protein and can be more specific in developing drugs that target it,” says the author.
The research team says it plans to capture more of ZAK’s structure, to get a deeper understanding of how the protein activates, and to find out what ZAK is doing when it’s not connected to a colliding ribosome.