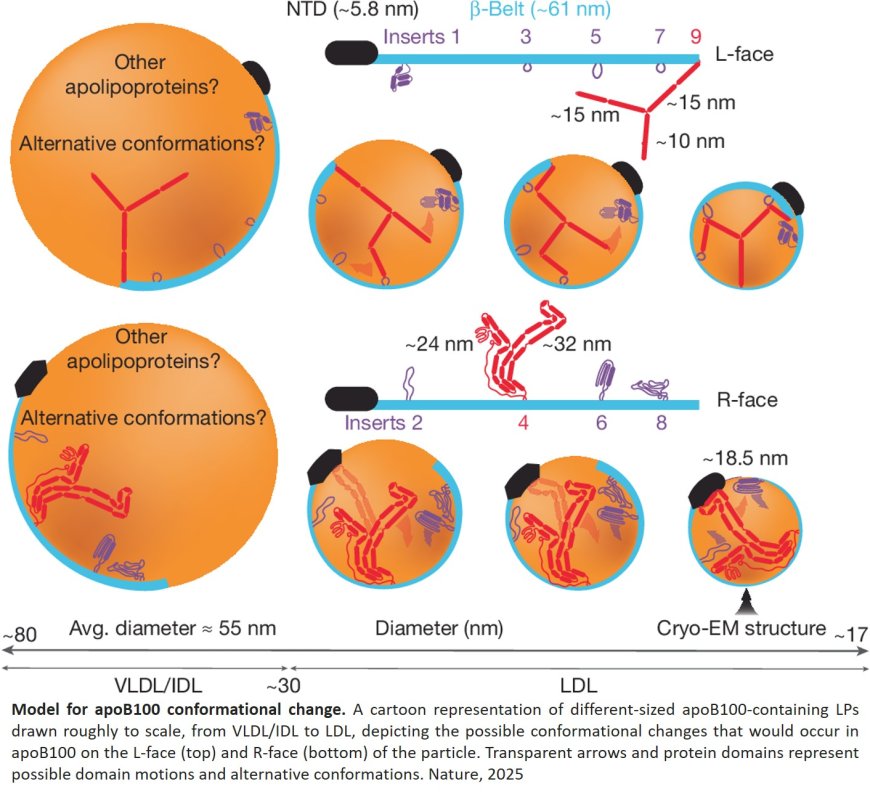
The structure of apolipoprotein B100 from human low-density lipoprotein
Low-density lipoproteins (LDL) — commonly known as bad cholesterol — have long been on scientists’ radar as a major contributor to heart disease. But these microscopic troublemakers have hidden their inner workings behind a maze of complexity. That is, until now.
In a new study published in Nature, the researchers have, for the first time, revealed the specific shape and structure of one of the body’s most important yet complicated proteins: ApoB100. Acting as a kind of molecular exoskeleton, this protein wraps around LDL particles, allowing them to travel through the bloodstream, researchers found.
The discovery may one day lead to the design of new drugs that target LDL, providing more precise treatments for high cholesterol and heart disease and potentially reducing the side effects of widely prescribed medications such as statin drugs.
“By integrating an AI neural network called AlphaFold with the cryo-electron microscopy images, we were able to get an even more detailed and higher-resolution picture of the structure of ApoB100. Ideally, this can lead to more targeted treatments that reduce heart disease-related risks without interfering with all the benefits cholesterol brings throughout the body.”
The structure consists of a large globular N-terminal domain and an approximately 61-nm-long continuous amphipathic β-sheet that wraps around the LDL particle like a belt. Distributed quasi-symmetrically across the two sides of the β-belt are nine strategically located interstrand inserts that extend across the lipid surface to provide additional structural support through a network of long-range interactions.
The authors further compared the structure to a comprehensive list of more than 200 intramolecular cross-links and find close agreement between the two.
The discovery not only helps researchers better understand fundamental aspects of fat and cholesterol metabolism, but it could also lead to the development of better, more specific testing and treatment for “bad” cholesterol, the author said.
“The most common ways of testing for cholesterol levels are currently not very specific,” the author said. “Going forward, if we can test for how many copies of this ApoB100 are in your blood, that would be a more accurate indicator of risk for heart disease.”