Circulating epigenetic signatures classifying brain insulin resistance in humans
If the brain no longer responds properly to insulin (insulin resistance), this can lead to overweight, diabetes, and Alzheimer's disease. Researchers have discovered small chemical modifications to genetic material (epigenetic changes) in the blood that indicate how well the brain responds to insulin. These markers could help to detect insulin resistance in the brain – by means of a simple blood test. The findings were published in the journal Science Translational Medicine.
“Insulin is not only involved in metabolism but also plays a key role in the brain with regard to cognitive functions, appetite regulation, and energy homeostasis,” explains the first author of the current publication. To date, the detection of brain insulin resistance remains cost and time-intensive, with no biomarkers currently available. “Our new study shows that we can extract epigenetic signatures from the blood that very precisely indicate whether the brain is still responding to insulin – or has stopped doing do,” says the senior author.
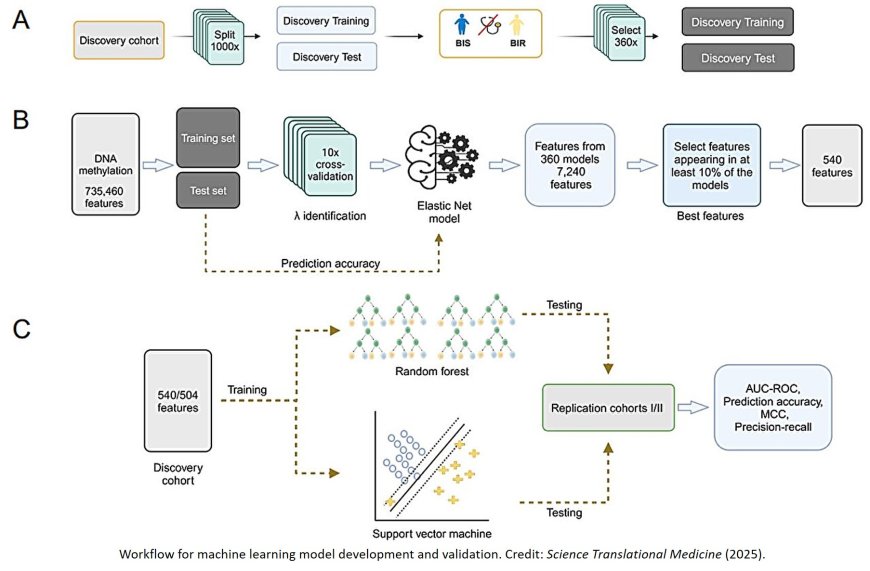
In order to identify epigenetic markers, the research team used a machine learning method to analyze DNA methylation patterns (small chemical modifications in DNA) in the blood. They studied people without type 2 diabetes (T2D) who differed in terms of their brain’s response to insulin but had similar peripheral insulin sensitivity values. Imaging data, metabolic data, and epigenetic data were integrated into the machine learning workflow.
In a first study cohort with 167 participants, the researchers identified 540 so-called CpG sites with methylation patterns that made it possible to reliably distinguish between people with and without insulin resistance in the brain.
“It is noteworthy that many of these methylation sites were associated with an elevated risk of T2D,” reports the senior author of the study. “This suggests a mutual influence between insulin resistance in the brain and metabolic disease.”
The findings were subsequently confirmed in two independent replication cohorts with 33 and 24 people – and with a high degree of accuracy (83% to 94%). “We were able to show that these signatures are reliable independent of age or BMI,” emphasizes the author.
All 540 examined CpG sites exhibited changed methylation patterns. For 98 of the identified CpG sites, the researchers found a correlation between blood methylation and brain methylation in a publicly available dataset. Many of the corresponding genes play a role in neuronal development, synapse formation, and signal transmission. “Our findings suggest that the epigenetic profile in the blood can reflect key processes in the brain,” explains the author.
Previous work had already shown that people with insulin resistance in the brain respond less well to lifestyle interventions, store more visceral fat, and experience more frequent cravings – all risk factors for the development of T2D.
“In future, the newly identified epigenetic markers could serve as a screening instrument in order to detect risk patients early on and provide them with targeted treatment – such as through more intensified lifestyle changes or new active substances ,” states the author
with conviction. “If we know who is insulin resistant in the brain, we can make interventions much more targeted and effective.” The team’s goal is now to develop a standardized test panel on the basis of the 540 identified CpG sites that can be used in clinical practice.
Whether epigenetic signatures in the blood can also be used for the early detection of neurodegenerative diseases such as Alzheimer’s disease remains a question for future studies.