Production of phosphorylated and functional αs1-casein in Escherichia coli
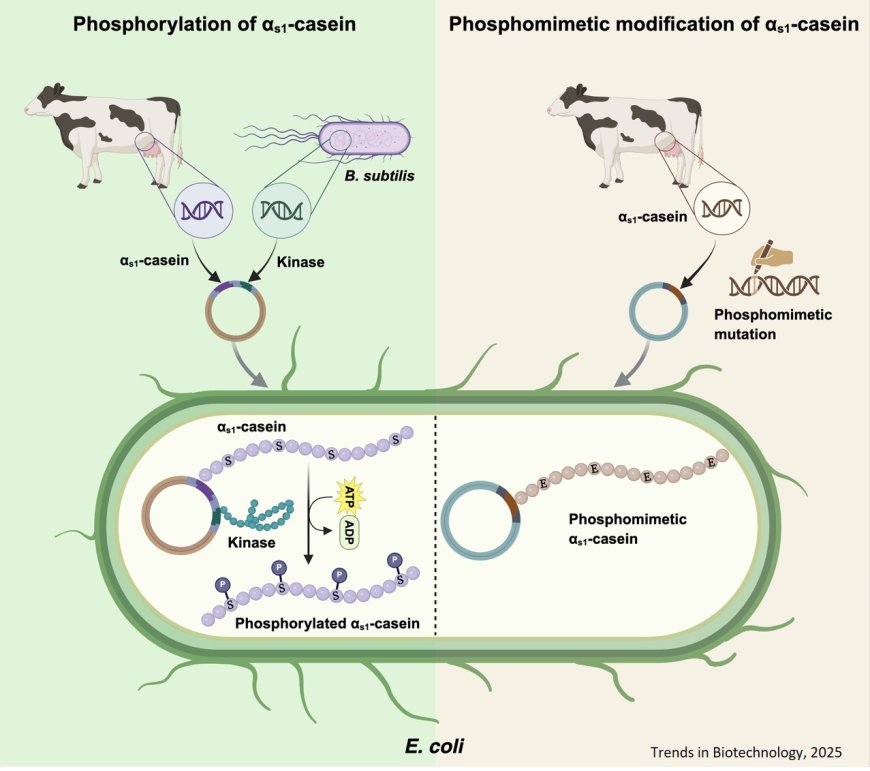
A major challenge in producing caseins in microbial systems is their reliance on essential post-translational modifications, particularly phosphorylation.
Phosphorylation of serine residues is critical for calcium binding and the formation of colloidal calcium phosphate nanoclusters, both of which are fundamental to casein micelle assembly and contribute to their functional and nutritional properties in dairy systems.
A phosphorylation pattern similar to bovine αs1-casein was achieved in recombinant αs1-casein through the use of Bacillus subtilis kinases PrkD and YabT.
Phosphomimetic casein offers a feasible alternative to phosphorylation while maintaining functional properties.
Both phosphorylated and phosphomimetic caseins exhibited significantly higher calcium-binding capacity compaared with unphosphorylated caseins.
https://www.cell.com/trends/biotechnology/fulltext/S0167-7799(25)00181-7