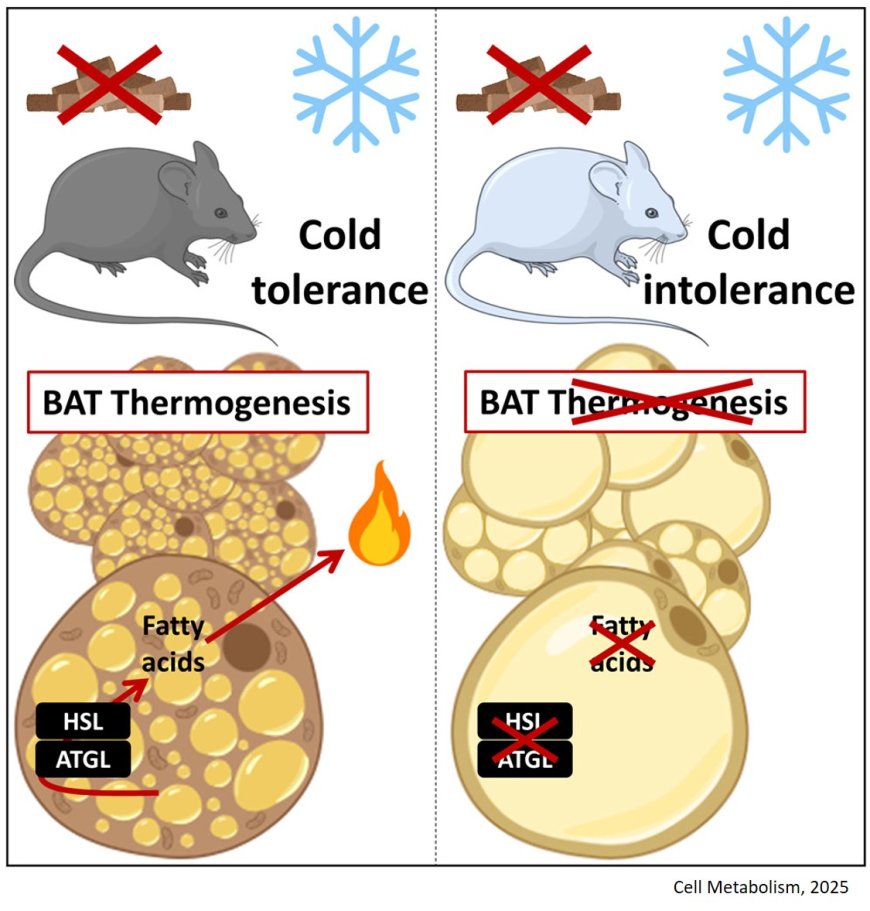
Neutral-lipase-mediated intracellular lipolysis in brown adipocytes to generate energy in cold-induced thermogenesis
Researchers have unveiled a protein supercomplex, termed SC-XL, that significantly boosts cellular energy production. Published in Cell Metabolism, this finding offers new avenues for therapeutic strategies, particularly in the treatment of ischaemic heart disease—a leading cause of death worldwide, where restricted blood flow diminishes oxygen supply to the heart.
A novel mechanism that enables cells to maintain energy production even when their main power supply drops/fails has been uncovered by biologists. The scientists found that a previously unknown protein supercomplex, dubbed SC-XL, enhances energy production and reduces harmful byproducts in cells with specific genetic disruptions. These findings, published in Cell Metabolism, could pave the way for new therapeutic approaches targeting disorders caused by deficiencies in cellular energy production, particularly ischaemic heart disease, a condition whereby narrowed arteries restrict blood and oxygen flow to the heart.
Mitochondria, often described as cellular power plants, generate energy through a sequence called the electron transport chain, comprising various protein complexes. This new research shows that SC-XL can enhance energy production even when traditional energy pathways falter, such as during ischaemic heart disease, which remains a leading cause of death in Singapore–accounting for nearly 19 per cent of deaths in 2019, and responsible for more than 20.5 million deaths globally each year, with the rates expected to rise by 2050 due to ageing populations and economic disparities.
The findings revealed that SC-XL formation can improve mitochondrial function even when Complex III activity is reduced by up to 70 per cent, helping sustain energy production and maintain heart function under ischaemic stress. By boosting fatty acid oxidation, SC-XL also improves metabolic health and reduces oxidative stress-induced damage.
The study’s first author, said the new findings could have exciting applications: “We’re optimistic about the potential of targeting supercomplexes as a therapeutic strategy for chronic metabolic diseases associated with mitochondrial dysfunction.”
Mitochondrial supercomplexes are thought to stabilise mitochondrial function during stress, helping maintain energy production and minimise cellular damage. Genetic mutations affecting the formation of Complex III can lead to the spontaneous creation of SC-XL. Using high-resolution cryo-electron microscopy, the researchers showed that SC-XL is composed of two Complex I subunits and two Complex III subunits. They found that the formation of SC-XL increases the number of folds in the inner mitochondrial membrane, expanding its surface area. As a result, more energy-producing proteins can be embedded in the membrane, boosting overall energy production. This structural adaptation enables cells to compensate for reduced Complex III activity, highlighting the dynamic and flexible nature of mitochondrial structure and function.
The team also found that if SC-XL formation was blocked in these cells, they couldn’t compensate for the diminished energy production. This underscores the critical role of SC-XL in maintaining the cellular energy balance.
The study’s lead author said: “Understanding supercomplexes transforms our approach to mitochondrial health. Although they have been observed biochemically for decades, their real functions have been elusive. We now see them as critical back-up systems that kick in to sustain energy when the primary sources falter. Our next challenge is to activate these back-up systems proactively, without waiting for a stress trigger.”
The team also found that the formation of SC-XL reduces the production of reactive oxygen species, which are harmful byproducts of mitochondrial activity that can damage cellular components. SC-XL seems to make cells use fats more efficiently as an energy source by enhancing fatty acid oxidation. This not only boosts energy production, but also helps prevent the accumulation of excess lipids, which can improve metabolic health.
https://www.cell.com/cell-metabolism/fulltext/S1550-4131(24)00414-5