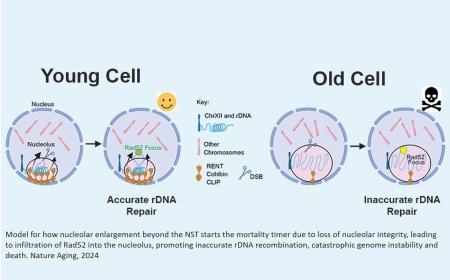
Peroxisome-mitochondria contact regulates mitochondrial stress
An international team has uncovered a surprising way compartments within cells work together to defend themselves against oxidative stress, a finding that could shift how we understand age-associated conditions such as diabetes and neurodegenerative diseases.
Published in Science, the study reveals a newly identified mechanism between two key compartments of the cell (mitochondria and peroxisomes) that helps manage internal stress and damage, highlighting an overlooked contributor essential to maintaining cellular health.
Mitochondria are often described as the powerhouses of the cell because they generate the energy needed for cells to function. This energy production also creates reactive oxygen species (ROS) as byproducts. These molecules can damage all parts of the cell, especially the mitochondria, and contribute to a condition called oxidative stress, an imbalance linked to diseases such as diabetes, neurodegeneration and cardiovascular conditions.
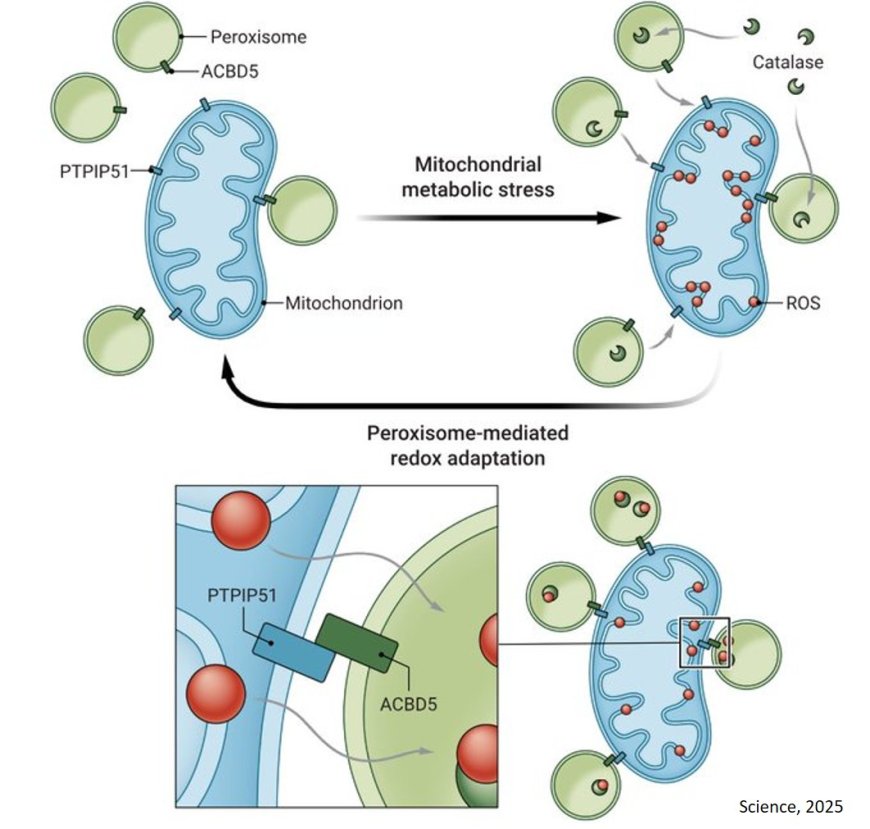
The international research team discovered a new mechanism by which mitochondria protect themselves from the harmful effects of ROS. The study found that ROS generated by mitochondria can move to another compartment of the cell called peroxisomes through a newly identified contact point between the two organelles.
Through this mechanism, peroxisomes can spare mitochondria from oxidative stress by acting as specialized sinks for ROS produced in mitochondria. This challenges the long-standing idea that cellular defense is confined within individual compartments and is the first to show movement of ROS between organelles as a defense mechanism.
"Conventional dogma has always been that ROS are independently processed by organelles, but this work challenges that assumption by showing a mechanism by which peroxisomes directly mitigate mitochondrial ROS,” says the lead author. “This discovery demonstrates that inter-organelle dynamics actively contribute to antioxidant defense, introducing a new perspective on how cells manage oxidative stress.”
The team identified that the contact site is formed by two proteins: PTPIP51 on mitochondria and ACBD5 on peroxisomes. These proteins create a bridge that allows ROS to move directly between the organelles, sparing mitochondria while preventing damage to other parts of the cell. Both PTPIP51 and ACBD5 are disease-relevant proteins and have previously been linked to neurodegeneration.
While ROS play an important role in the body, too much can damage healthy cells, and too little can prevent the body from eliminating harmful ones, like cancer cells. By identifying the bridge that allows ROS to move between mitochondria and peroxisomes, the team has uncovered a spatially specific mechanism that helps maintain this delicate balance.
The discovery presents not only a potential new target for therapies but also challenges the assumption behind broad-spectrum antioxidant therapies. Targeting the precise cellular location where ROS are regulated, such as peroxisomes, may be key to developing more effective treatments.
“We know that dysregulation of mitochondrial ROS is linked to many diseases, so identifying these proteins that bring the two organelles together gives us a new entry point to explore how we might restore or strengthen this protective mechanism in cells,” says the senior scientist.