Linking early life epigenetic memory to adult brain inflammation
Why do some people remain healthy through childhood yet become more vulnerable to brain disorders such as dementia later in life?
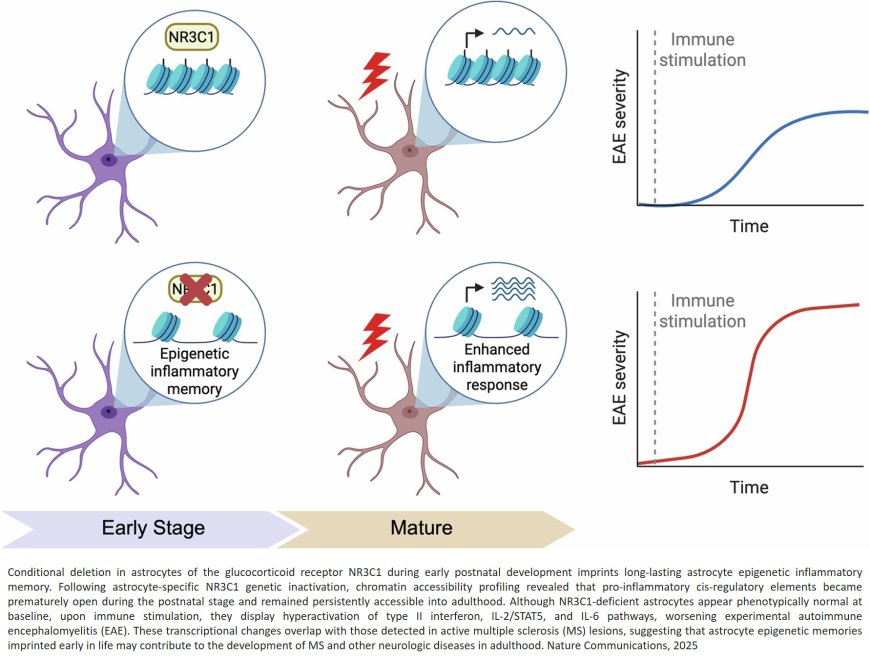
A research team has uncovered a key part of the answer: a developmental ‘switch’ in astrocytes—the brain’s most abundant support cells that shapes how strongly the brain’s immune system reacts in adulthood. The study identifies a gene, NR3C1 (encoding the glucocorticoid receptor), as a master regulator of this switch and shows how early-life epigenetic ‘memory’ can predispose the adult brain to excessive inflammation.
In mouse models, the researchers mapped gene-regulatory programs across multiple stages of astrocyte development and found that NR3C1 acts during a brief early-postnatal window to enforce long-term immune restraint.
To build this map, the team combined state-of-the-art 3D epigenome profiling with RNA sequencing and chromatin accessibility analyses, capturing how DNA folds and which regulatory elements contact target genes. They identified 55 stage-specific transcription factors that guide astrocyte maturation; among them, NR3C1 emerged as the critical ‘switch’ in early life. Notably, deleting NR3C1 in astrocytes did not disrupt normal development. However, when the adult mice were challenged with an autoimmune model of multiple sclerosis, animals lacking astrocytic NR3C1 mounted exaggerated inflammatory responses and developed more severe disease.
Mechanistically, the study shows that early loss of NR3C1 epigenetically primes immune genes - keeping their regulatory elements open and ready - so that later in life these genes respond too strongly to inflammatory cues. In effect, NR3C1 serves as an early ‘brake’ that prevents over-activation of astrocyte immune programs in adulthood.
“This is the first demonstration that astrocyte immune functions are governed by epigenetic memory,” said the senior author. “Our findings offer new clues to the origins of degenerative brain disorders, including Alzheimer’s disease.”
“We reveal a temporal regulatory window in astrocyte development that can set the stage for disease vulnerability in adulthood,” added the author. “Understanding the 3D genome logic behind these programs could open paths to therapies for immune-related brain disorders such as multiple sclerosis.”
The results of this study were published in the journal Nature Communications.
https://www.nature.com/articles/s41467-025-64102-w
https://sciencemission.com/astrocyte-epigenetic-inflammatory-memory