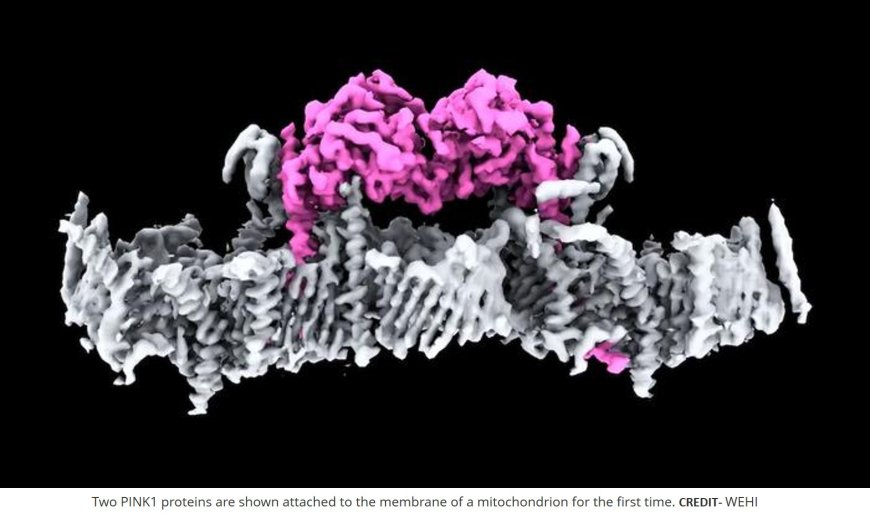
Electron microscopy of PINK1 attachment to mitochondrial membrane
The researchers have made a huge leap forward in the fight against Parkinson’s disease, solving a decades-long mystery that paves the way for development of new drugs to treat the condition.
First discovered over 20 years ago, PINK1 is a protein directly linked to Parkinson’s disease – the fastest growing neurodegenerative condition in the world. Until now, no one had seen what human PINK1 looks like, how PINK1 attaches to the surface of damaged mitochondria, or how it is switched on.
In a major breakthrough, researchers have determined the first ever structure of human PINK1 bound to mitochondria, in findings published in Science. The work could help find new treatments for the condition that currently has no cure or drug to stop its progression.
Parkinson’s disease is insidious, often taking years, sometimes decades to diagnose. Often associated with tremors, there are close to 40 symptoms including cognitive impairment, speech issues, body temperature regulation and vision problems.
Mitochondria produce energy at a cellular level in all living things, and cells that require a lot of energy can contain hundreds or thousands of mitochondria. The PARK6 gene encodes the PINK1 protein, which supports cell survival by detecting damaged mitochondria and tagging them for removal.
In a healthy person, when mitochondria are damaged, PINK1 gathers on mitochondrial membranes and signals through a small protein called ubiquitin, that the broken mitochondria need to be removed. The PINK1 ubiquitin signal is unique to damaged mitochondria, and when PINK1 is mutated in patients, broken mitochondria accumulate in cells.
Although PINK1 has been linked to Parkinson’s, and in particular Young Onset Parkinson’s Disease, researchers had been unable to visualise it and did not understand how it attaches to mitochondria and is switched on.
Corresponding author on the study, said years of work by his team have unlocked the mystery of what human PINK1 looks like, and how it assembles on mitochondria to be switched on.
“This is a significant milestone for research into Parkinson's. It is incredible to finally see PINK1 and understand how it binds to mitochondria,” said the author.
“Our structure reveals many new ways to change PINK1, essentially switching it on, which will be life-changing for people with Parkinson’s.”
Lead author on the study, said PINK1 works in four distinct steps, with the first two steps not been seen before.
First, PINK1 senses mitochondrial damage. Then it attaches to damaged mitochondria. Once attached it tags ubiquitin, which then links to a protein called Parkin so that the damaged mitochondria can be recycled.
“This is the first time we’ve seen human PINK1 docked to the surface of damaged mitochondria and it has uncovered a remarkable array of proteins that act as the docking site. We also saw, for the first time, how mutations present in people with Parkinson’s disease affect human PINK1,” said the author.
The authors determined a 3.1-Å resolution cryo-electron microscopy structure of dimeric human PINK1 stabilized at an endogenous array of mitochondrial TOM and VDAC complexes.
Symmetric arrangement of two TOM core complexes around a central VDAC2 dimer is facilitated by TOM5 and TOM20, both of which also bind PINK1 kinase C-lobes.
PINK1 enters mitochondria through the proximal TOM40 barrel of the TOM core complex, guided by TOM7 and TOM22.
The structure explains how human PINK1 is stabilized at the TOM complex and regulated by oxidation, uncovers a previously unknown TOM-VDAC assembly
The idea of using PINK1 as a target for potential drug therapies has long been touted but not yet achieved because the structure of PINK1 and how it attaches to damaged mitochondria were unknown.
The research team hope to use the knowledge to find a drug to slow or stop Parkinson’s in people with a PINK1 mutation.
One of the hallmarks of Parkinson’s is the death of brain cells. Around 50 million cells die and are replaced in the human body every minute. But unlike other cells in the body, when brain cells die, the rate at which they are replaced is extremely low.
When mitochondria are damaged, they stop making energy and release toxins into the cell. In a healthy person, the damaged cells are disposed of in a process called mitophagy.
In a person with Parkinson’s and a PINK1 mutation the mitophagy process no longer functions correctly and toxins accumulate in the cell, eventually killing it. Brain cells need a lot of energy and are especially sensitive to this damage.