Role of glutamine in plant root microbial colonization
When we talk about microbiota, we usually think of the one inhabiting our gut. But there is another, less known and equally vital: the plant microbiota. In an article featured on the cover of Science (a research team unveil the subtle alliances and rivalries that unfold between bacteria and roots, hidden beneath the soil.
The plant microbiota, or “phytobiome,” brings together communities of bacterial and fungal microorganisms that can be partners, allies—and sometimes enemies. The part most closely associated with roots is called the “rhizospheric” microbiome, from greek “rhizo-“ (root). To assemble specialized and protective microbiome, plants selectively recruit these bacteria from the soil. The fragile balance of the microbial community influences the plant’s growth, health, and ability to withstand environmental stress. When plants are weakened, some microbes can even switch roles and become pathogens.
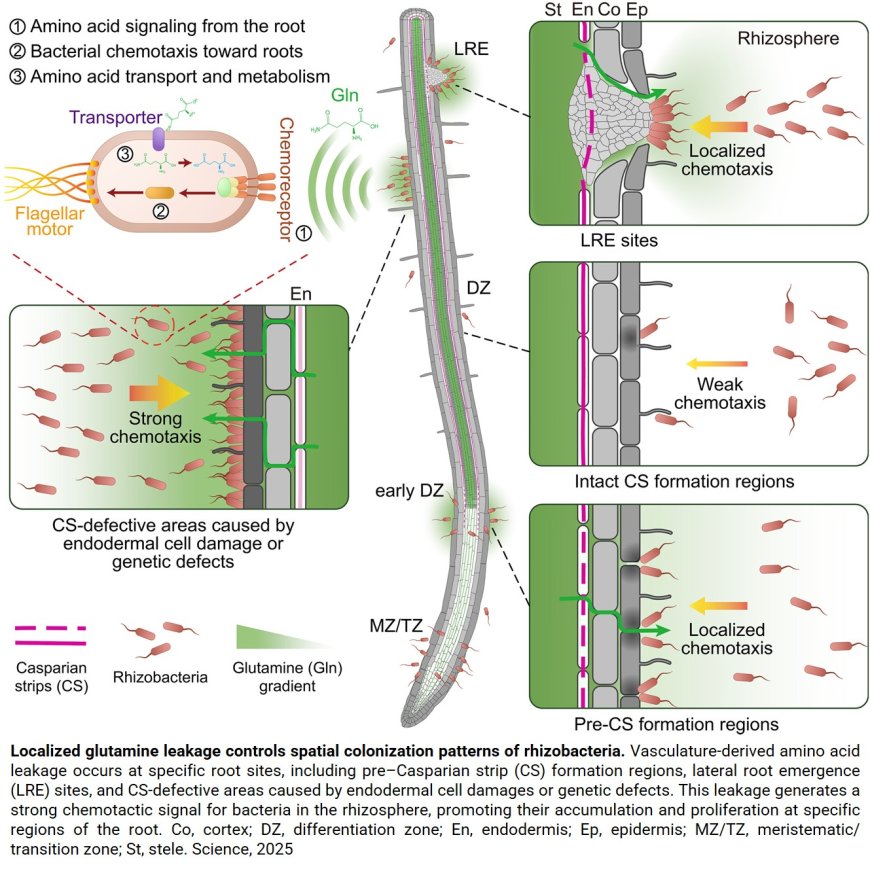
How do plants choose their microbial partners? By releasing a complex cocktail of molecules called “root exudates.” These exudates contain sugars, amino acids, and other organic compounds. Whereas it was known that these compounds are of great importance for bacterial colonization, little was known about how, where, and when exudates are released at the microscale relevant to microorganisms. This is the puzzle researchers set out to solve,
Much like the intestinal epithelium in animals, the plant root endodermis acts as a selective filter, preventing the leakage of energy-rich compounds from their central transporting vein into the soil. But during growth, this barrier can temporarily break: “For example, when a lateral root emerges from the main root, part of the barrier breaks down to allow the radicle to come through,” explains a co-senior author of the article.
“Although the broken barrier will soon be repaired, the rupture causes a temporary outflow. Bacteria then cluster and proliferate precisely at that spot. The question was: what attracts them and makes them proliferate?” From this came the scientists’ hypothesis: Alteration of the endodermal barrier influences microbial recruitment and the composition of the bacterial communities. The challenge was to uncover the mechanism behind this phenomenon. To do so, mutants of the model plant Arabidopsis thaliana (thale cress), completely lacking endodermal barriers, were used. “Our observations confirmed that changes in endodermal barriers profoundly affect bacterial colonization,” says the co-author. “We therefore wondered whether the bacteria were especially fond of one or more particular substances that were leaking”. Making use of their Arabidopsis mutants, the team then discovered a significant accumulation of amino acids—especially glutamine—in the exudates.
Glutamine plays an important role in transporting nitrogen from root to shoots and was a prime candidate for the researchers. To see whether this bacterium is attracted to glutamine, the researchers genetically manipulated this model bacterium: “We generated bacteria that had specifically lost their ability to ‘sense’ glutamine. Intriguingly, these bacteria were unable to find the sites where lateral roots were emerging.” reports a co-first author of the study. Moreover, the researchers were also able to observe that the bacteria use glutamine for their growth, by developing a fluorescence reporter system that only switches on when glutamine is metabolized. This amino acid thus acts as a major signal allowing bacteria to find and colonize precise leakage sites on the root surface. “We showed that the bacteria metabolically adapt to this glutamine-rich niche and use it as an energy source, which enables them to proliferate even more,” adds the author.
These findings demonstrate that localized glutamine leaks shape bacterial colonization and highlight the fine-tuned interactions between roots and microbes. Thr team now aims to identify other attractive compounds, especially those released under stress conditions (drought, salinity, heat).
Could such discoveries be applied to agriculture, at a time when reducing fertilizers and pesticides is a priority? “This is the dream of many researchers. Yet each soil has its own unique microbiota, making it difficult to ensure that a specific bacterial strain will take hold and protect a given plant.” warns the author. Laboratory experiments are needed to uncover general principles of interaction between roots and bacteria, using simplified microbial communities. “What is certain,” he concludes, “is that plant health depends on their microbiota. Without better knowledge of their interactions with roots, we will never truly understand what happens in our fields.”