Predicting if multiple sclerosis medication will be effective for patients
The researchers have developed a tool that can predict how patients will respond to natalizumab, one of the most commonly used drugs for treating multiple sclerosis.
Despite its effectiveness, about 35% of users do not respond completely to therapy and experience a return of symptoms within two years of starting treatment. Additionally, while it helps reduce the frequency and severity of outbreaks and slows disease progression, it can cause adverse reactions such as an increased risk of serious infection (progressive multifocal leukoencephalopathy), headaches, muscle and stomach pain, fatigue, and depression.
Using an innovative methodology, the group of scientists achieved an important advance in precision medicine. This advance will allow patients to have a better quality of life in the future with targeted treatments that have fewer side effects and produce positive results in shorter periods of time. It will also reduce costs for the public health system.
The drug acts as an antibody, blocking the binding of the immune system protein VLA-4 to a molecule called VCAM-1. This prevents immune cells from entering the brain and causing inflammation. After treatment, immune system cells, such as CD8+ T cells, become more rounded. This change is linked to remodeling of actin, a protein that primarily promotes cell support but also plays a role in the movement, shape, and interaction of cells with each other and their surroundings.
Using high-content imaging (HCI), the scientists discovered that poor outcomes with natalizumab treatment are associated with distinct actin remodeling responses in CD8+ T cells and their ability to elongate, even when under the influence of the drug. The cells become misshapen and more longitudinal. These findings were published in the journal Nature Communications.
“The results are important because they can contribute to an improvement in patients’ quality of life, avoiding unnecessary side effects and treatment delays, as well as optimizing costs, the article’s first author said.
Multiple sclerosis (MS) is an autoimmune, inflammatory, and degenerative neurological disease that affects the central nervous system, leading to motor, cognitive, and mental disorders. Symptoms range from loss of muscle strength and difficulty walking to memory impairment, attention difficulties, and mood swings. It is estimated that 2.8 million people worldwide have MS, including approximately 40,000 in Brazil. Most diagnoses occur in young adults between the ages of 20 and 50, and women are two to three times more likely than men to be affected.
High-content cell imaging (HCI) combines advanced microscopy technology with automated image analysis to extract multiple pieces of information per cell, such as shape and size, organelle distribution, protein localization, response to drugs, and genetic disorders. HCI has most often been used for cancer studies.
Adopting this type of analysis was a step forward compared to other personalized medicine studies, which generally use cytometry (analysis of the physical and chemical characteristics of cells), serology, or transcriptomics (evaluation of how DNA information is transcribed into RNA and used to produce proteins and other molecules).
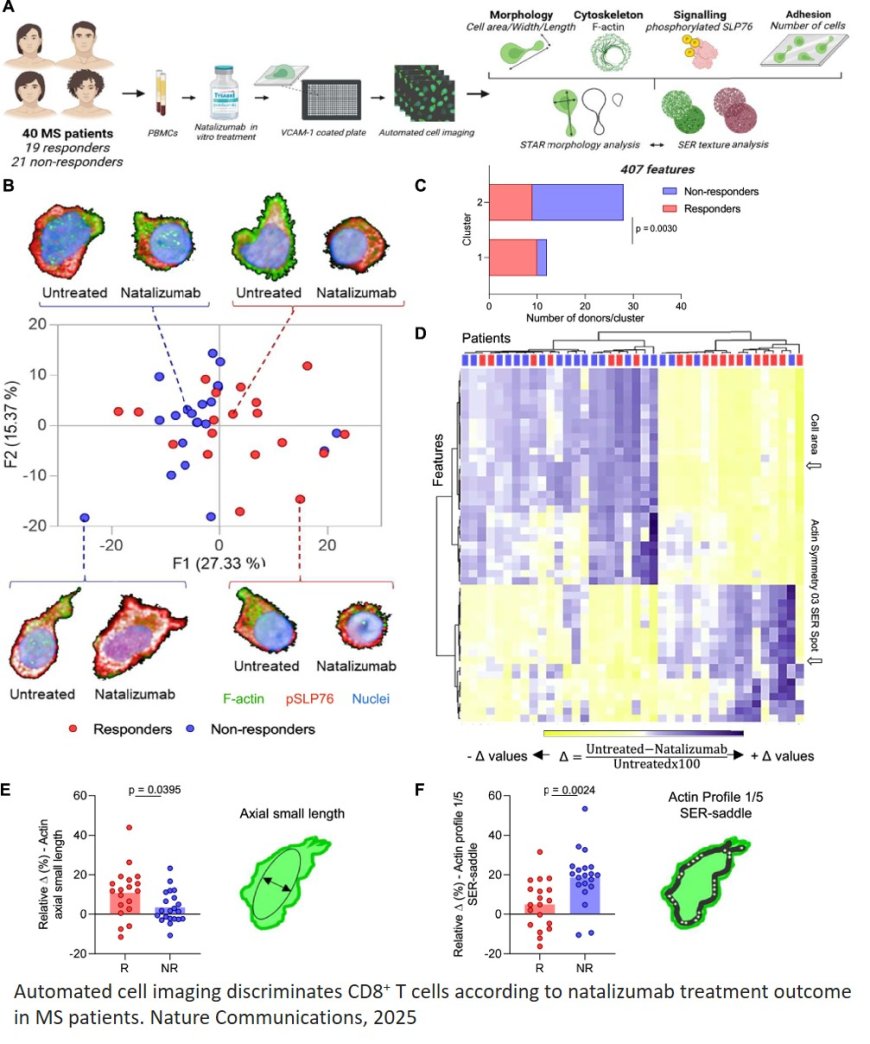
In the study, the researchers began by applying natalizumab in vitro to blood cells, including T cells, from patients with MS who had not yet been treated with the drug. The cells were stimulated via VLA-4 and seeded on VCAM-1-coated plates. The samples were from individuals linked to institutions in France.
More than 400 morphological profile characteristics were extracted, such as area, width/length ratio, and actin organization. Of these characteristics, 130 presented information relevant to the research. Using machine learning, the researchers then created more than one million combinations.
The study achieved 92% accuracy in the discovery cohort and 88% in the validation cohort in predicting clinical response to natalizumab treatment. CD8+ T cells proved to be a relevant subpopulation for this prediction. Non-responding patients exhibited a more resistant actin remodeling profile, characterized by reduced loss of polarity and increased migration capacity. This suggests that maintaining the migratory state of CD8+ T cells may compromise treatment efficacy.
https://www.nature.com/articles/s41467-025-60224-3
https://sciencemission.com/profiling-of-T-cells-predicts-clinical-response