Gut microbiota acts like an auxiliary liver
Microbes in the mammalian gut can significantly change their hosts’ amino acid and glucose metabolism, acting almost like an extra liver, according to a new preclinical study by investigators.
The study, published in Cell Host & Microbe, adds to the growing list of ways in which the microbiome influences physiology, and could lead to new strategies to treat conditions such as inflammatory bowel disease and diabetes.
In recent years, scientists have found that the billions of microbes living on and in the human body profoundly influence our physiology. Senior author wanted to take a deeper look at how the essential microbes in the gut affect our access to the nutrients extracted from the food we’ve ingested.
“They ‘eat’ before us, taking first dibs on the nutrients from the food we consume, and leaving us with what remains after they satisfy their own nutritional needs,” said the author.
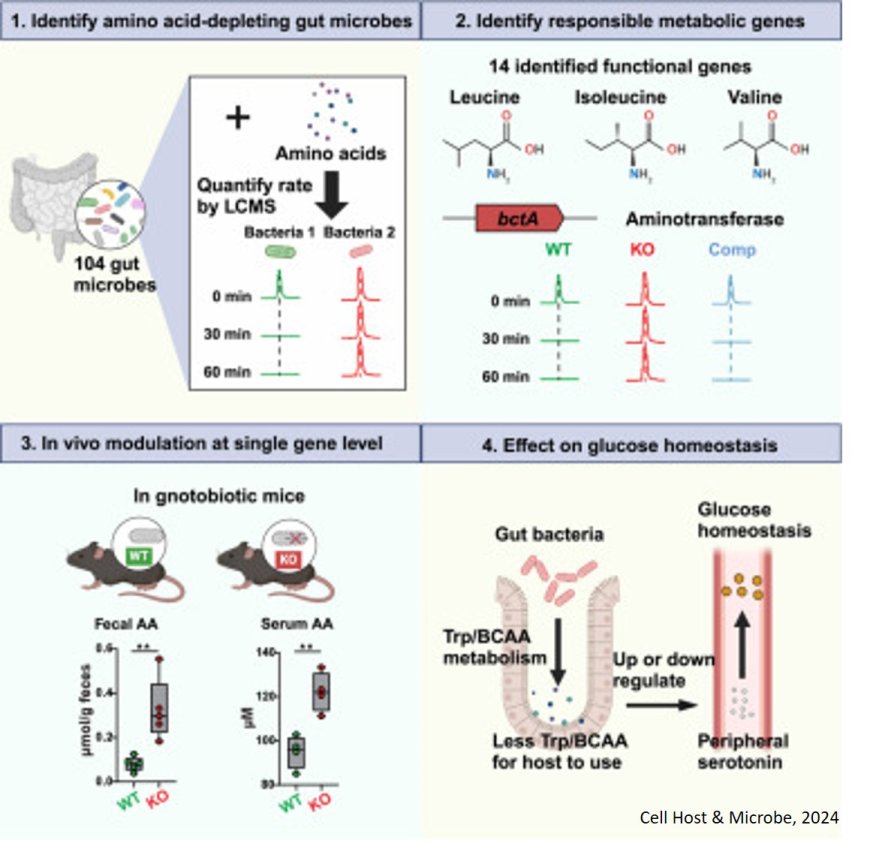
To better understand this process, the authors assessed how efficiently different bacteria that naturally inhabit our intestines, called human gut commensals, deplete amino acids, the building blocks of proteins. Due to the poorly characterized metabolic functions of many gut bacteria, the team experimented with various settings to find the optimal conditions for their study. After screening more than 100 different human gut microbes, the investigators pinpointed several that are highly efficient at metabolizing various dietary amino acids. When these microbes colonized the gastrointestinal tracts of germ-free mice—mice that initially had no microbes—the levels of those amino acids dropped in the host's intestine and bloodstream.
The team then identified the specific bacterial metabolic genes that deplete amino acids. It was a long list. “We found that in one single bacterium, there are over 20 different genes encoding a similar enzymatic function,” the author said. “And because we have improved our CRISPR-Cas9 gene deletion techniques for gut bacteria, we were able to perform a large gene deletion screen and identify the metabolic genes in the bacteria that were responsible for depleting amino acids."
The scientists took their findings from cultured cells into animals, giving germ-free mice genetically modified strains of bacteria, one at a time. “We can now precisely manipulate individual genes for depleting amino acids in the gut,” the author said. “This allows us to assess the individual function of these genes and to see how they actually impacts host amino acid homeostasis.”
That work produced a surprising result; by consuming a specific class of amino acids, gut microbes can alter their hosts’ blood glucose homeostasis. Further analysis revealed that by changing amino acid availability, the microbes appear to affect the production of the neurotransmitter serotonin, which in turn changes glucose regulation.
“A lot of these metabolic functions can be done by the liver, but now we’ve found that there are functionally comparable enzymes encoded by the gut microbiota that can do the same or similar things,” said the author. “It’s like there is a second liver operating in the gut.”
The team is now designing new strategies to modulate the bacterial enzymes more precisely, and looking at how various combinations of bacteria affect the host’s amino acid metabolism.
Tantalizingly, some of the same genes the current study highlights are also dysregulated in the gut microbiomes of patients with digestive and metabolic diseases. Drugs targeting specific microbial genes, or engineered strains of bacteria, could potentially provide new ways to treat such conditions, according to the author.
“These metabolic genes might be potential biomarkers for diseases like type 2 diabetes or inflammatory bowel disease, and they are also potential treatment targets,” he added. "Our research demonstrates the possibility of precisely manipulating gut microbiota to regulate host metabolism and improve host metabolic functions.”