How hair stem cells under stress make decisions to graying or melanoma
Throughout life, our cells are constantly exposed to environmental and internal factors that can damage DNA. While such DNA damage is known to contribute to both aging and cancer, the precise connection—particularly how damaged stem cells shape long-term tissue health—has remained elusive.
Melanocyte stem cells (McSCs) are tissue-resident stem cells that serve as the source of mature melanocytes, the pigment-producing cells responsible for hair and skin coloration. In mammals, these stem cells reside in the bulge–sub-bulge region of hair follicles as immature melanoblasts, maintaining pigmentation through cyclical regeneration.
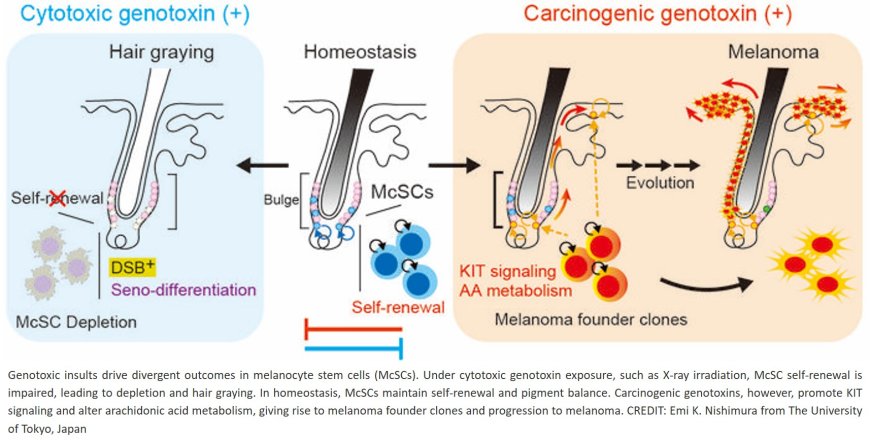
Published in the journal Nature Cell Biology, the study used long-term in vivo lineage tracing and gene expression profiling in mice to investigate how McSCs respond to different types of DNA damage. The team identified a specific response to DNA double-strand breaks: senescence-coupled differentiation (seno-differentiation), a process in which McSCs irreversibly differentiate and are then lost, leading to hair graying. This process is driven by activation of the p53–p21 pathway.
In contrast, when exposed to certain carcinogens, such as 7,12-dimethylbenz(a)anthracene or ultraviolet B, McSCs bypass this protective differentiation program—even in the presence of DNA damage. Instead, they retain self-renewal capacity and expand clonally, a process supported by KIT ligand secreted both from the local niche and within the epidermis. This niche-derived signal suppresses seno-differentiation, tipping McSCs toward a tumor-prone fate.
The senior author says, “These findings reveal that the same stem cell population can follow antagonistic fates—exhaustion or expansion—depending on the type of stress and microenvironmental signals.” The author adds, “It reframes hair graying and melanoma not as unrelated events, but as divergent outcomes of stem cell stress responses.”
Importantly, this study does not suggest that graying hair prevents cancer, but rather that seno-differentiation represents a stress-induced protective pathway that removes potentially harmful cells. Conversely, when this mechanism is bypassed, the persistence of damaged McSCs may predispose to melanomagenesis.
By identifying the molecular circuits that govern this fate bifurcation, the study provides a conceptual framework that links tissue aging and cancer, and highlights the beneficial role of eliminating potentially harmful stem cells through natural "senolysis," resulting in a phenotype that safeguards against cancer.