Why does female fertility decline with age?
The ticking of the biological clock is especially loud in the ovaries — the organs that store and release a woman’s eggs. From age 25 to 40, a woman’s chance of conceiving each month decreases drastically.
For decades, scientists have pointed to declining egg quality as the main culprit. But new research shows that the story is bigger than the eggs: The surrounding cells and tissues of the ovary play a crucial role in how eggs mature and how quickly fertility wanes.
“We’ve long thought of ovarian aging as simply a problem of egg quality and quantity,” said the senior author of the study, which appears in Science. “What we’ve shown is that the environment around the eggs — the supporting cells, nerves, and connective tissue—is also changing with age.”
Understanding these changes may hold the key not only to extending fertility, but also to improving health. The risks of many age-related diseases rise after menopause or ovary removal, and slowing ovarian aging could help reduce these risks.
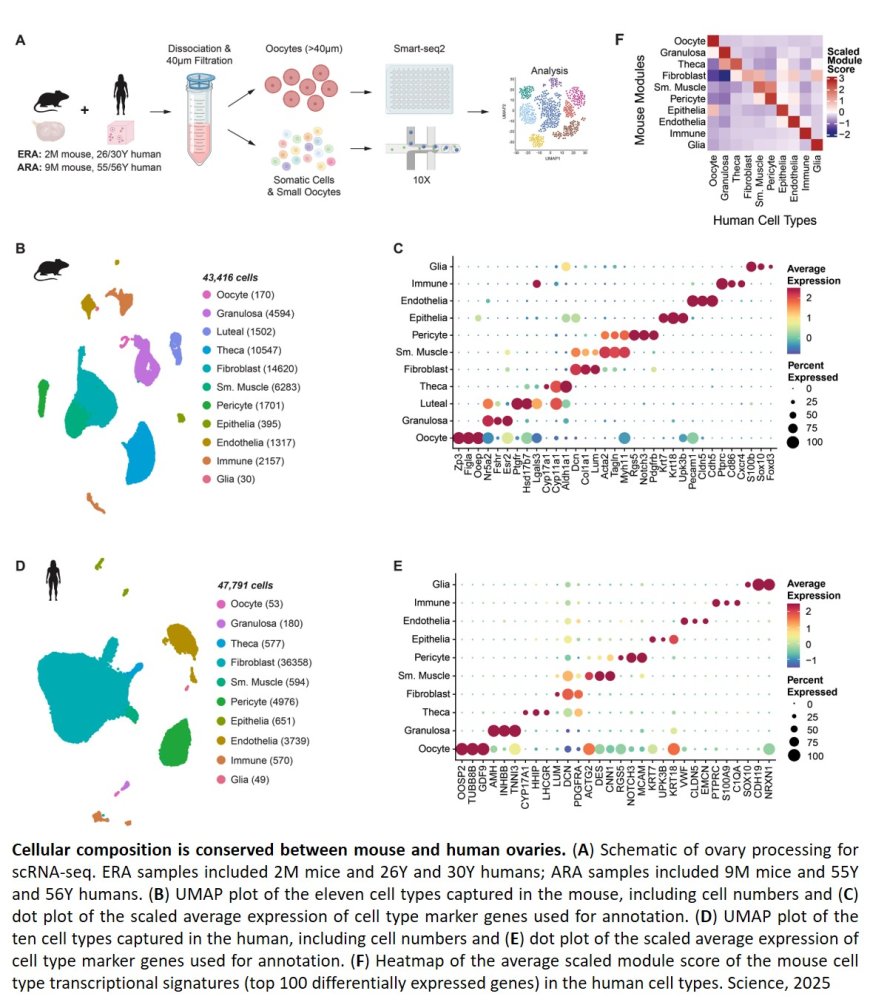
The researchers set out to profile what normal aging looks like in the ovaries of mice and humans. First, they developed a new three-dimensional imaging technique that allowed them to visualize eggs in the ovaries without having to slice the organs into thin layers, as had been done before.
In mice that were the equivalent of 30 to 40 human years, they observed a dramatic drop in both immature resting eggs that are waiting in reserve and in growing eggs that are beginning to mature for ovulation. And just like women in their 30s, the mice did not conceive easily with in vitro fertilization (IVF).
When the scientists extended their 3-D imaging to human ovaries, they uncovered an unexpected finding: Eggs are not evenly scattered throughout the ovary. Instead, they cluster in “pockets” surrounded by egg-free zones. With age, the density of eggs within these pockets declines.
“This was a surprise — we assumed eggs would be distributed more evenly based on what we see in the developing ovary,” said the author. “These pockets suggest that even within one ovary, the environment around an egg may influence how long it lasts and how well it matures.”
Next, the researchers wanted to study what genes were active in ovary cells as they aged. Ovarian tissue from humans is hard to come by, and eggs are large and incredibly fragile. So, instead of using standard miniature devices that separate and tag cells to sequence their active genes, the group painstakingly isolated individual eggs by hand to separate them from other cells.
After studying nearly 100,000 mouse and human cells, they identified 11 major cell types found in the ovaries, including one surprise: Glia, a type of support cell typically associated with nerves and most extensively studied in the brain, were in the ovaries.
At the same time, the study revealed that sympathetic nerves — the same nerves involved in the “fight or flight” response — form dense networks in ovaries that become even more dense with age. When the researchers ablated these nerves in mice, the animals had more eggs in reserve but fewer that matured, suggesting the nerves help decide when eggs start growing. Together, the observations on glia and sympathetic nerves suggest a new role for the nervous system in ovarian health.
Other support cells called fibroblasts also changed with age, triggering inflammation and scarring in the ovaries of women in their 50s — years earlier than such scarring appears in organs like the lungs or liver.
“This all points to a brand-new line of inquiry about how nerves, blood vessels, and other cell types communicate with eggs,” the author said. “It tells us that ovarian aging is not just about the egg cells but about their whole ecosystem.”
For researchers, one of the most important takeaways of the new work is the similarity between human and mouse ovaries.
“Until now, it was somewhat unclear whether we could use mice as a model for humans when it comes to the ovaries — we have quite different reproductive windows,” the author said. “But the similarities we saw in this study make us confident that we can move forward in mice and apply those lessons to humans.”
In addition, the new roadmap of healthy ovaries over time offers a starting place to ask how ovarian aging changes in different situations. The team is already launching studies probing whether some drugs could change the timing or speed of ovarian aging, the author said. Ultimately, they hope to uncover ways to slow or delay ovarian aging, to impact both fertility and other diseases, like cardiovascular disease, which are common in women after menopause.
“The fountain of youth may actually be the ovary,” said the co-first author of the study. “Delaying ovarian aging could promote healthier aging overall.”
https://www.science.org/doi/10.1126/science.adx0659
https://sciencemission.com/human-and-mouse-ovaries-across-age