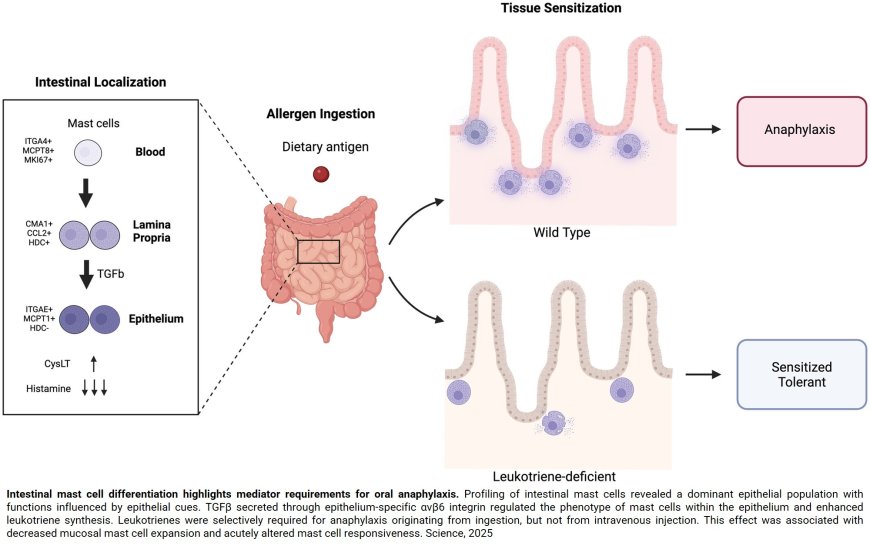
Cysteinyl leukotrienes stimulate gut absorption of food allergens to promote anaphylaxis
A drug already FDA-approved for asthma was found to nearly eliminate life-threatening allergic reactions to food allergens in mice — a breakthrough that could lead to new protection for millions of people living with food allergies, reports a new study.
The findings will be published in the journal Science.
The discovery came after the scientists identified, in mice, a previously unknown role for a gene called DPEP1, which they found is essential in regulating anaphylaxis, a rapid and potentially fatal allergic reaction.
By using the asthma drug Zileuton to block the pathway involving this gene, the scientists nearly eliminated allergic responses in mice that were previously highly susceptible to food-induced anaphylaxis. The mice were given peanut extract orally shortly after receiving Zileuton while the team monitored for symptoms.
“It was actually shocking how well Zileuton worked,” said co-senior study author.
“After treatment with Zileuton, 95% of the mice showed almost no symptoms of anaphylaxis. The treatment reversed their risk from 95% susceptible to 95% protected,” added the co-senior author.
The discovery of the new pathway came after a yearslong forward genetic screen, a process where scientists breed generations of mice to narrow down the specific genes responsible for biological differences, such as susceptibility to food allergy. Once the scientists found that the DPEP1 gene controlled leukotrienes in the gut — inflammatory molecules already targeted by asthma drugs — they tested Zileuton, an FDA-approved drug that blocks their production.
The authors show that reduced DPEP1 activity causes leukotriene (LTC4 and LTD4) accumulation in the gut, acting on the CysLT receptors 1 and 2 (CysLTR1 and CysLTR2).
This promotes food allergen transport through goblet cells to the submucosa and bloodstream, where IgE-loaded mast cells are activated to trigger anaphylaxis.
Food allergies are on the rise and affect more than 33 million people in the U.S. — nearly one in 10 people. Yet predicting an allergic individual’s risk of anaphylaxis and preventing severe reactions from accidental exposure remains challenging.
Currently, there are only two FDA-approved treatments for certain food allergies — and no cure. One is an oral immunotherapy for peanut allergy, which doesn’t work for everyone and can itself trigger anaphylaxis. The other is a costly injection (omalizumab) that also isn’t effective for all allergic patients.
Zileuton could offer a new approach: a simple pill that temporarily shields allergic individuals by blocking the body’s anaphylactic pathway before it activates.
“This is a totally different, out-of-the-box approach to treat food allergy, unlike anything we’ve tried before,” the author said. “We’ve seen tragic, even fatal reactions from hidden ingredients like ground peanuts in a sauce. For parents sending their child to a birthday party, or for anyone flying where they can’t control what’s being served, this could be a powerful protective drug.”
The team launched a small early-stage clinical trial in July to test whether blocking this newly identified pathway with Zileuton in humans is as effective as it was in mice.
The findings also shed light on a long-standing puzzle in allergy medicine: why some individuals test positive for food allergens but experience no symptoms when they eat the food.
“Let’s say you’re told you’re allergic to peanuts based on a blood test, but you’ve eaten peanuts your whole life without any problems,” the author said. “This pathway we discovered may be one explanation for why some of those people are protected.”
That group has been a challenge for clinicians and a source of stress for patients, the author added, because current diagnostic tests only estimate allergy risk, not tolerance.
“Our findings open a whole new area for future research into how people develop food allergies in the first place, and why some react while others don’t,” the author said.
The breakthrough wouldn’t have been possible without long-term investment in scientific research, the author said.
“If you’d asked me five or six years ago to guess the pathway that would lead to this discovery, I never would have picked this gene or the leukotriene molecules,” the author said.