Polysaccharide assembly and transport in Pseudomonas aeruginosa biofilm
Biofilms are conglomerates of bacteria and other organisms, which are feared in medicine as well as other areas because they can contain pathogens and are highly resistant to treatment. Chemists examined how the polysaccharide “Pel” – a central component of many biofilms – is exported out of the cell by the pathogen P. aeruginosa. In the scientific journal Nature Communications, they describe the structure of the so-called PelBC export complex, which represents the last station in the cell before “Pel” is released.
Bacterial biofilms are common aggregates of cells, which ensure the survival of microorganisms in harsh environments and are highly resistant to mechanical and chemical treatments. They can present a danger to humans where they e.g. cover surfaces of medical devices or in the food industry and harmful organisms can colonise them.
Biofilms enable bacteria to exchange genomic information with each other. This makes it easier for them to develop resistance to antibiotics – when resistant bacteria pass on resistance information to other species. They also facilitate nutrient uptake.
In particular where pathogenic bacteria form biofilms, it is important to understand which mechanisms play a role in their formation. The research group have focused on the pathogen Pseudomonas aeruginosa (for short: P. aeruginosa), which can cause e.g. pneumonia, urinary tract infections and meningitis in humans. The pathogen is resistant to multiple antibiotics and a key so-called “hospital germ”.
The senior author: “In order to form biofilms, the bacteria synthesise and export various biopolymers, above all polysaccharides – specific sugar chains. Despite decades of research, knowledge of how the synthesis or transport takes place remains limited. We have concentrated on the polysaccharide ‘Pel’, which is produced by P. aeruginosa.”
The production of Pel requires a protein machinery comprising several sub-units, which crosses two membranes. The author: “To date, however, neither the structure of the machinery nor its dynamics were known. With the help of cryo-electron microscopy, we can resolve the structure of the PelBC complex in the lipid membranes and identify the path of the Pel polysaccharide.”
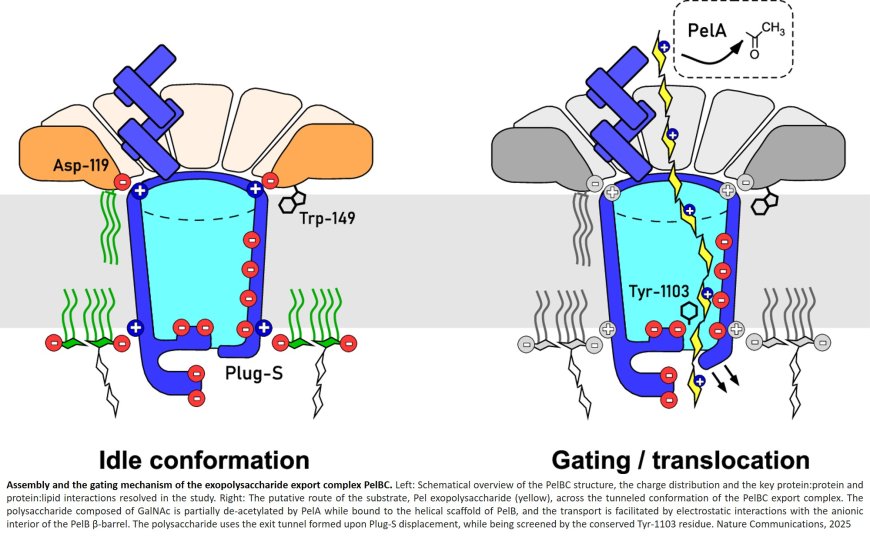
The authors reveal the architecture of the outer membrane complex PelBC for Pel export, where the essential periplasmic ring of twelve lipoproteins PelC is mounted on top of the nanodisc-embedded β-barrel PelB. The PelC assembly is stabilized by electrostatic contacts with the periplasmic rim of PelB and via the membrane-anchored acyl chains.
The negatively charged interior of the PelB β-barrel forms a route for the cationic Pel exopolysaccharide. The β-barrel is sealed at the extracellular side, but molecular dynamic simulations suggest that the short loop Plug-S is sufficiently flexible to open a tunnel for the exopolysaccharide transport.
The lead author, adds: “Our study shows how electrostatic interactions are used in nature to assemble the export complex and enable the transport of the polysaccharide. However, for the cell to survive, the pore cannot remain permanently open for transport. A small conformational change at the end of this pore is therefore necessary – a tiny gate is opened, so to speak.”
With a view to the prospects for application, the author comments: “It is fascinating to see the organisation of a complex structure in such detail. Among other things, it shows how nature solves challenges in protein design, for example the coupling of a symmetrical ring of PelC sub-units to the asymmetrical PelB channel. It may be possible to use our results to block the export of Pel in a targeted manner and thus suppress biofilm formation.”
In the next step, the research group intends to examine this export process in detail. Another involved protein complex, which is responsible for the synthesis of Pel in the cytoplasm of the cell and its transport across the inner membrane, will also be examined in greater detail.