Protein shapeshifting in necroptotic cell death signaling
Recent work has shed light on additional layers of regulation: post-translational modifications, proteolytic cleavage, and sequestration of key necrosomal machinery can attenuate necroptosis.
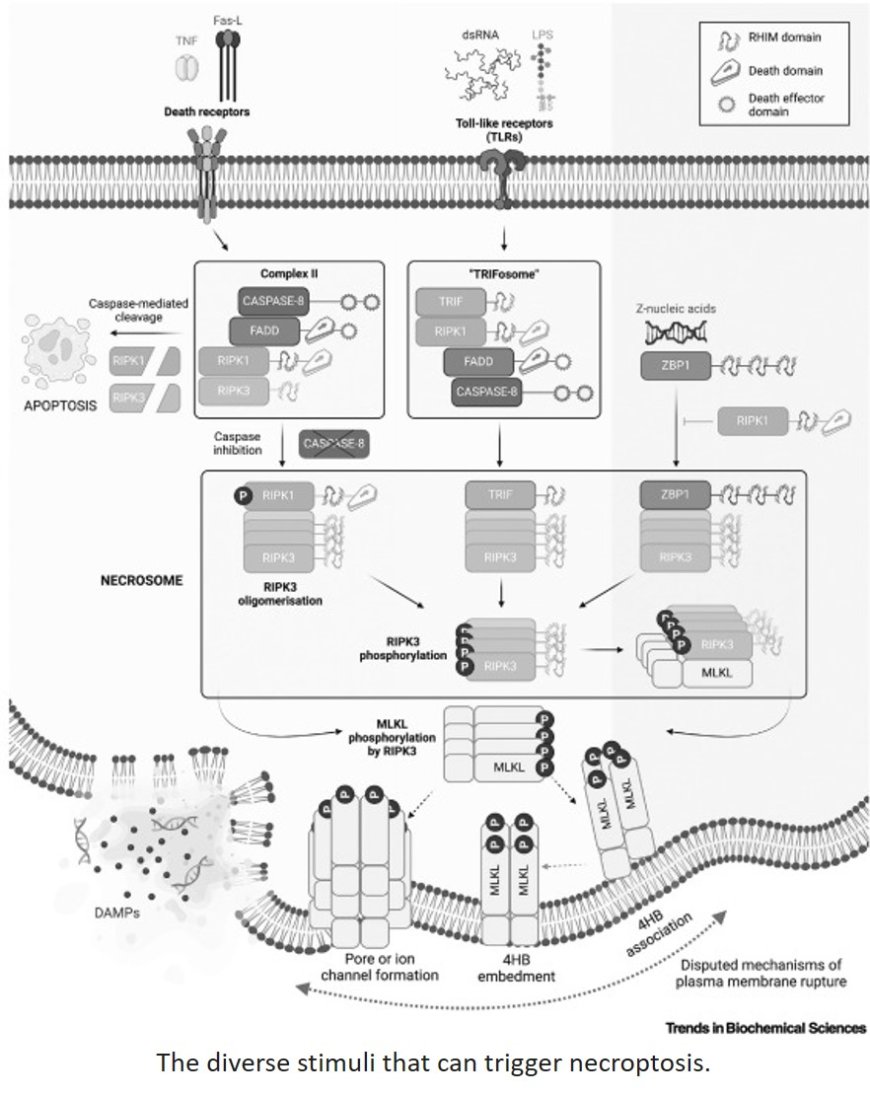
Death receptors and pathogen sensors, including the emerging player, Z-DNA-binding protein-1 (ZBP1), trigger necroptosis via convergent receptor interacting protein kinase-3 (RIPK3)- mediated mixed lineage kinase domain-like (MLKL) phosphorylation at the necrosome.
Recent biophysical studies of RIPK1, TIR domain-containing adapter-inducing IFN-β (TRIF), or ZBP1 and RIPK3 RIP homotypic interaction motif (RHIM– RHIM) interactions suggest hierarchies in homo- and hetero-oligomerization at the heart of necrosome formation.
The role of necroptotic effector conformation in pathway modulation has emerged from detailed structural studies, especially of the terminal executioner, MLKL.
Lipidation has emerged as a promoter of MLKL membrane targeting and cell lysis, indicating that diverse, and as yet undiscovered, post-translational modifications will regulate necroptosis.
https://www.cell.com/trends/biochemical-sciences/abstract/S0968-0004(24)00258-5
https://sciencemission.com/Protein-shapeshifting