How sperm achieves energy demands for fertilization
The scientists have pinpointed the molecular “switch” that supercharges sperm for their final sprint to an egg — a breakthrough that could reshape infertility treatments and pave the way for safe, nonhormonal male contraceptives.
“Sperm metabolism is special since it’s only focused on generating more energy to achieve a single goal: fertilization,” said the senior author of the paper.
Before ejaculation, mammalian sperm rest in a low-energy state. Afterward, as they swim through the female reproductive tract, they undergo a series of changes that ultimately help them reach and fertilize an egg. These include swimming with quick, vigorous movements, as well as a change to the membranes that will encounter an egg.
“Many types of cells undergo this rapid switch from low to high energy states, and sperm are an ideal way to study such metabolic reprogramming,” said the author.
Metabolism is similarly essential for sperm function, and while scientists knew that behavioral changes prior to fertilization required a large amount of energy, they weren’t sure how sperm adjusted to meet the demand — until now.
The group created a special technique that allowed them to track the metabolism of glucose, which sperm take up from their environment and use as a sort of fuel.
By tracing the chemical journey of glucose within sperm, they observed key differences between dormant and active specimens.
“You can think of this approach like painting the roof of a car bright pink and then following that car through traffic using a drone,” the author explained.
“In activated sperm, we saw this painted car moving much faster through traffic while preferring a distinct route and could even see what intersections the car tended to get stuck at,” the author added.
The study paints a fuller picture of the high-energy, multistep process required for sperm to reach their goal of fertilization.
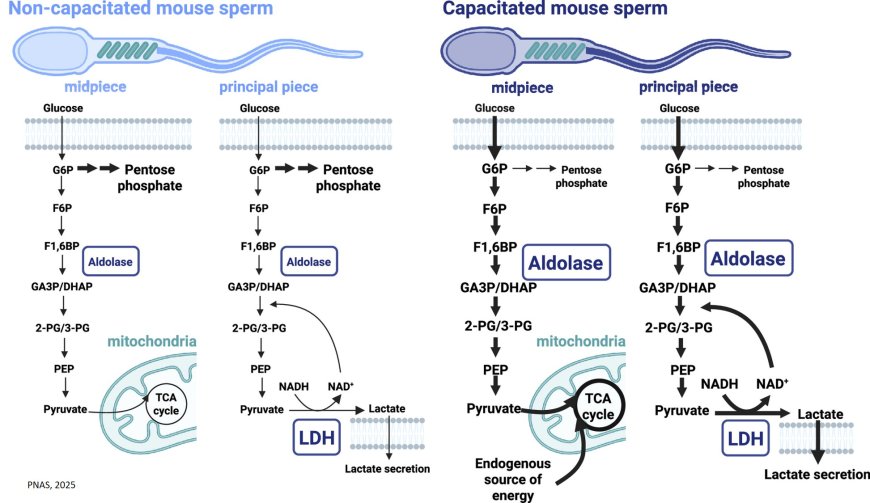
This includes the discovery that a particular enzyme called aldolase helps sperm convert glucose into energy and that sperm even utilize molecular fuel that they already have on board when they begin their trek.
The experiments also revealed how some enzymes regulate the flow of glucose like traffic controllers.
In the mitochondria-containing midpiece, glycolytically generated pyruvate feeds the tricarboxylic acid (TCA) cycle to further maximize energy yield via oxidative phosphorylation. In the mitochondria-free principal piece of the flagellum, pyruvate produced from glycolysis is reduced to lactate by lactate dehydrogenase, which also serves to regenerate oxidized nicotinamide adenine dinucleotide (NAD+) ensuring a sufficient supply to support glycolysis.
The resultant lactate is at least partially secreted. Finally, we find evidence that there is an as yet unknown endogenous source of energy in sperm, feeding the upregulation of TCA cycle intermediates.
Looking ahead, the author will continue to explore how sperm use a variety of fuel sources like glucose and fructose to meet their energy needs. This research can potentially impact a number of reproductive health issues.
With one in six individuals impacted by infertility globally, the author sees the analysis of sperm metabolism as an especially promising research direction for improving both assisted fertility techniques and the diagnosis of infertility in patients.
This work can also help develop new methods of contraception like nonhormonal birth control.
“Better understanding the metabolism of glucose during sperm activation was an important first step, and now we’re aiming to understand how our findings translate to other species, like human sperm,” the author said.
“One option is to explore if one of our ‘traffic-control’ enzymes could be safely targeted as a nonhormonal male or female contraceptive,” the author added.
The traditional development of male contraceptives has focused on blocking sperm at their creation. This approach, however, comes with notable drawbacks. The process of becoming infertile is far from on demand, and such contraceptives are commonly hormone-based, leading to many severe side effects.
The latest findings from the group are paving the way for a sperm metabolism-centered solution to these challenges: an inhibitor-based, nonhormonal method of contraception that would allow for on-demand male infertility with little to no side effects.
“Right now, about 50% of all pregnancies are unplanned, and this would give men additional options and agency in their fertility,” the author said. “Likewise, it creates freedom for those using female birth control, which is hormone-based and highly prone to side effects.
“I’m excited to see what else we can find and how we can apply these discoveries.”
The work appeared in the journal Proceedings of the National Academy of Sciences.