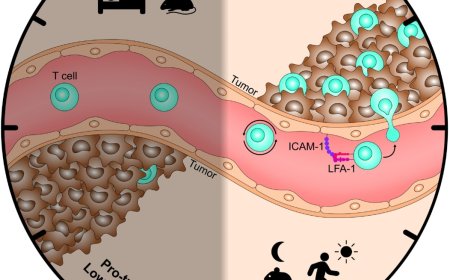
Tumor-infiltrating lymphocyte (TIL) therapy
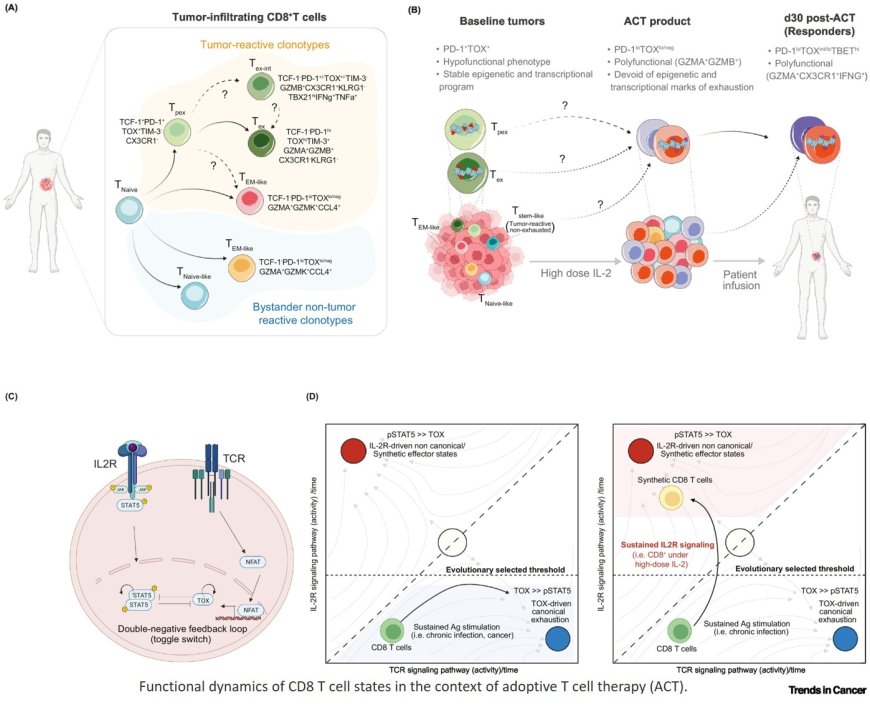
The US Food and Drug Administration (FDA) approval of lifileucel marks a milestone for tumor-infiltrating lymphocyte (TIL) therapy in solid tumors.
New single-cell and multi-omic technologies reveal functional heterogeneity of tumor-reactive TILs.
Identification of clinical and molecular biomarkers improves patient selection and treatment optimization.
Novel expansion methods, engineered TILs, and adoptive cell therapy (ACT) combinations are overcoming key resistance mechanisms.
Improved understanding of the tumor microenvironment (TME) informs strategies to extend TIL therapy beyond melanoma.
https://www.cell.com/trends/cancer/fulltext/S2405-8033(25)00200-6