How neural stem cells are activated in the adult human brain
A research team reveal important new insights into the activation dynamics of neural stem cells (NSCs). These are the stem cells that build our central nervous systems and the self-renewing.
The collaborative team aimed to shed light on how neural stem cells integrate a multitude of signals from different cell types in the brain – and how they decode these signals.
These are big questions because how NSCs react to signals in their cellular environment controls whether they remain in their dormant, non-dividing state known as “quiescence” or if they get activated to grow and divide, generating new neurons and glia in the process.
The findings published in Cell Stem Cell, will certainly be of deep interest to scientists studying a range of adult neurological diseases and aging. In the neural landscape, rousing NSCs from dormancy, where they conserve resources and energy, is key for neural regeneration and brain injury repair.
“These data make it possible to understand better how NSCs can be activated to generate more neurons and glia in order to counteract different neurological disorders and aging. We are currently studying NSCs’ responses for some of these conditions,” says the senior author. (Neurogenesis is the process by which new neurons are formed in the brain.)
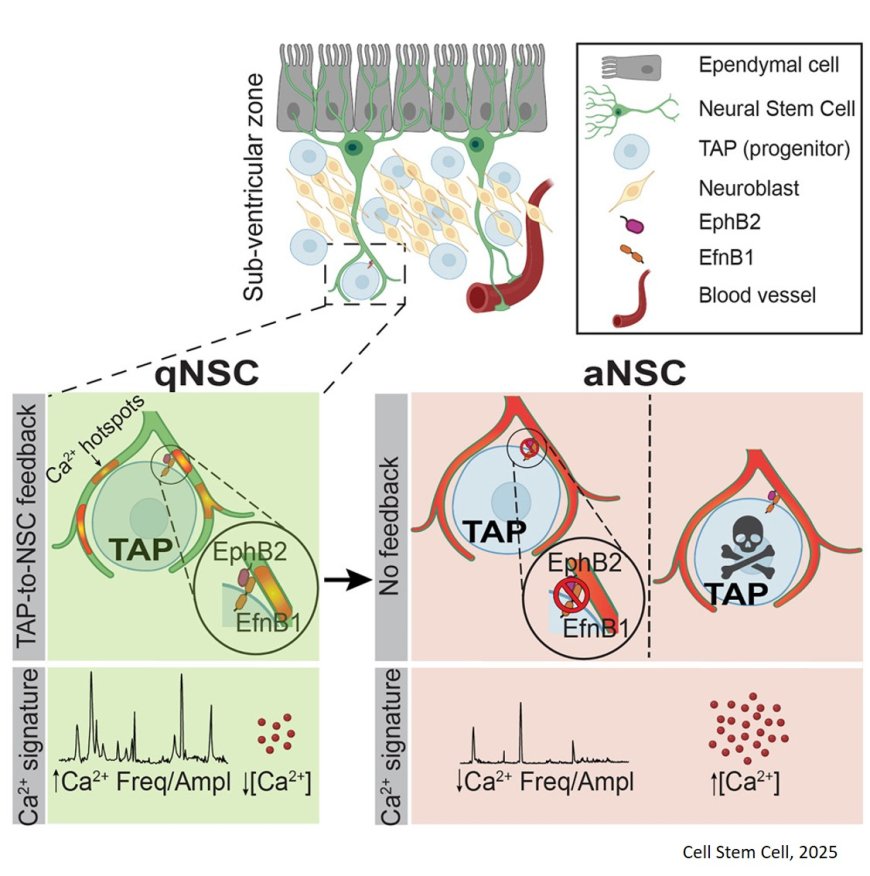
One avenue of new insight focuses on how stem cells wrap around progeny called “daughter” cells – genetically identical cells created after a parent cell divides.
The team discovered that neural stem cells are in fact receiving constant feedback from their chatty daughter cells. The author likens this to a “parent-child relationship” in which the parent is closely attuned to their child’s feedback.
“Many parents will relate to this since parents like to receive news, or feedback, from their children. Based on this feedback, the parents will either stay reassured that everything is going well or take an action,” the author says, comparing this dynamic to the cellular state of quiescence or activation.
Revealing this hidden mechanism is a major finding because it provides an entirely new framework for how to understand this cellular relationship in the human brain.
“Until now it was thought that NSCs only generate progeny and that there is no interaction between them,” the author says. “But our work challenged this notion and showed that there is tight structuro-functional interaction between NSCs and their progeny – and that the number of progeny or the efficiency of progeny-NSC interaction determines whether neural stem cells stay quiescent or get activated to generate neurons and glia.”
In a nutshell, the research team found that a low number of daughter cells leads to activation of neural stem cells, while a large number of offspring keeps them in their typical state of quiescence in the adult brain.
Further, the new study advances our understanding of how NSCs integrate and decode a multitude of signals in space and time. The senior author says the research unveils for the first time that “calcium signaling in NSCs allows for integration and decoding of all these signals.”
These new windows of understanding into how NSCs decode signals and how their activation is triggered offers strong potential for informing any future treatments for human neurodevelopmental disorders. Indeed, advancing this potential is the next step for the research collaborators as they explore questions suggested by this work.
“We are now exploring how interactions of NSCs with different cell types in their micro-environment is affected in different physiological and pathological conditions as well as in healthy aging,” says the author.
https://www.cell.com/cell-stem-cell/abstract/S1934-5909(25)00001-3