A novel subset of adipose macrophages identified in obesity
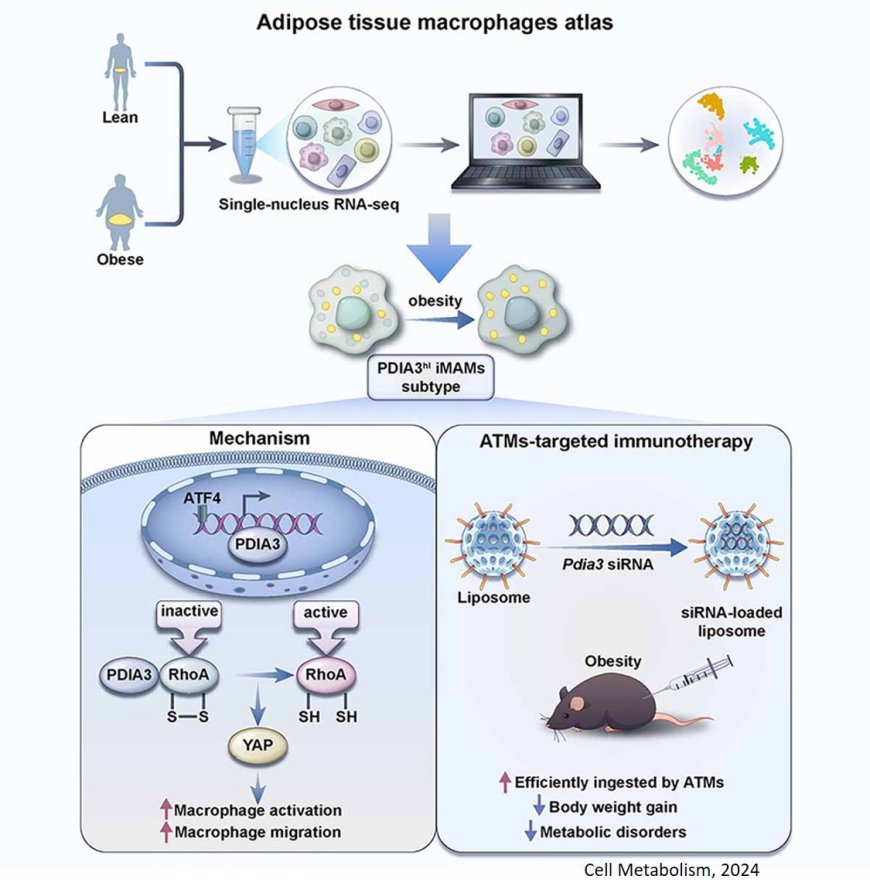
Adipose tissue homeostasis requires adipose tissue macrophages (ATMs) and thus identification of the authentically pathogenic ATM subpopulation under obese setting is important.
The researchers identified a distinct ATF4hiPDIA3hiACSL4hiCCL2hi inflammatory and metabolically activated macrophage (iMAM) subpopulation in obese adipose tissues. They require PDIA3 for their maintenance, migratory and pro-inflammatory properties.
Mechanistically, ATF4 serves as a metabolic stress sensor to transcribe PDIA3, which then imposes a redox control on RhoA activity and strengthens the pro-inflammatory and migratory properties of iMAMs through RhoA-YAP signaling.
Administration of Pdia3 small interfering RNA (siRNA)-loaded liposomes effectively repressed adipose inflammation and high-fat diet (HFD)-induced obesity. Thus, strategies aimed at targeting PDIA3 could be a viable approach against obesity and metabolic disorders.
https://www.cell.com/cell-metabolism/fulltext/S1550-4131(24)00361-9