Amyloid precursor protein in Alzheimer's disease
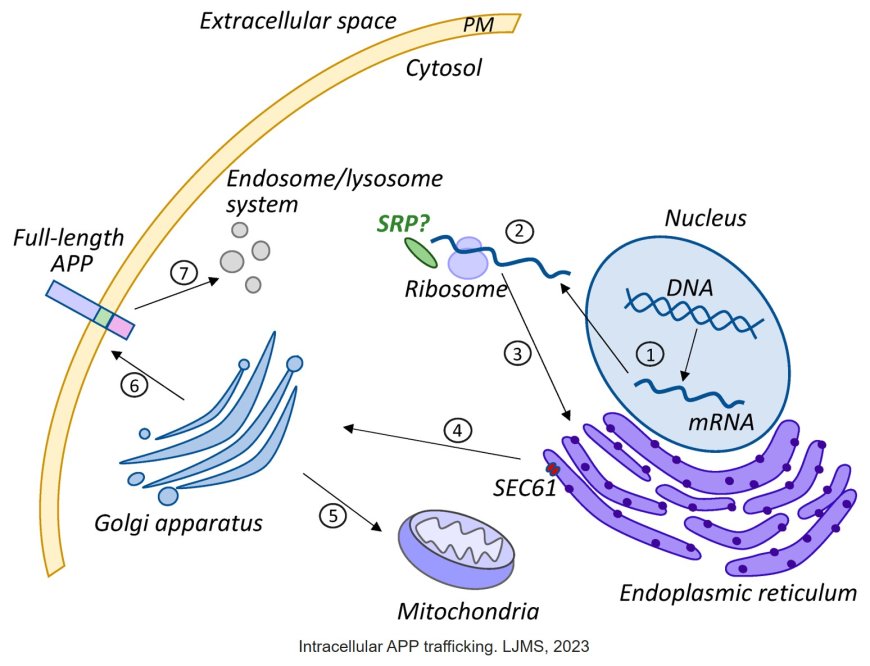
Amyloid precursor protein (APP) is a transmembrane protein that plays a crucial role in the pathogenesis of Alzheimer's disease (AD). Here's an overview of APP biology:
Structure and Function
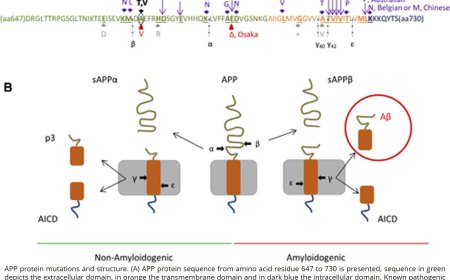
1. Domain structure: APP consists of a large extracellular domain, a transmembrane domain, and a short intracellular domain.
2. Functions: APP is involved in various cellular processes, including:
1. Cell adhesion and migration
2. Neuroprotection and neurotrophic support
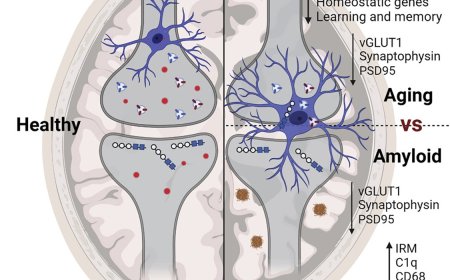
3. Regulation of synaptic plasticity and neurotransmission
Processing and Cleavage
1. α-Secretase cleavage: APP is cleaved by α-secretase (ADAM10 or ADAM17) within the extracellular domain, releasing the soluble APPα (sAPPα) fragment.
2. β-Secretase cleavage: APP is cleaved by β-secretase (BACE1) near the transmembrane domain, releasing the soluble APPβ (sAPPβ) fragment.
3. γ-Secretase cleavage: The remaining APP fragment is cleaved by γ-secretase (a complex of presenilin, nicastrin, Aph1, and Pen2) within the transmembrane domain, releasing the amyloid-β (Aβ) peptide.
Amyloid-β (Aβ) Formation
1. Aβ aggregation: The Aβ peptide is prone to aggregation, forming oligomers, protofibrils, and eventually, amyloid fibrils.
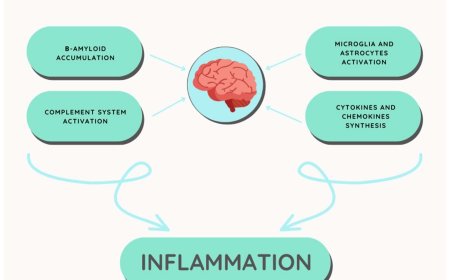
2. Aβ deposition: Aβ fibrils deposit in the brain, forming senile plaques, which are a hallmark of Alzheimer's disease.
Implications in Alzheimer's Disease
1. APP mutations: Certain mutations in the APP gene can lead to early-onset familial Alzheimer's disease.
2. Aβ accumulation: The accumulation of Aβ peptides is thought to contribute to neurodegeneration and cognitive decline in Alzheimer's disease.
3. Therapeutic targets: APP and Aβ have been identified as potential therapeutic targets for Alzheimer's disease, with several treatments aiming to reduce Aβ production or promote its clearance.
https://www.mdpi.com/1422-0067/24/19/14794