Aging-related blood disorders from supercharged mitochondria
As we age, blood stem cells, the essential source of new blood cells in the body, can accumulate genetic mutations. These mutations can give the cells a growth advantage, laying the foundation for developing serious health conditions. Now, scientists have not only discovered the mechanism that fuels their unchecked growth but have also found a way to stop it.
The study reported in Nature Communications reveals that a common aging-associated mutation in the gene Dnmt3a boosts the power-generating function of mitochondria in blood stem cells. This mutation allows the cells to make copies of themselves more readily than normal and creates fertile ground for the development of clonal hematopoiesis, a condition that dramatically increases the risk of heart disease, blood cancers, and other illnesses.
Although clonal hematopoiesis develops silently with age— more than half of all 80-year-olds are estimated to be affected by the condition — the mutated blood stem cells can produce inflammatory molecules that disrupt blood production and weaken the immune system.
“This work gives us a new window into how and why blood stem cells change with age and how that sets up an increased risk of diseases like cancer, diabetes, and heart disease,” said the author. “It also points toward a new opportunity to intervene and potentially prevent age-associated conditions not only in the blood but everywhere the blood touches.”
Building on the previous work the team knew that Dnmt3a is frequently mutated in blood stem cells with aging as well as in blood cancers. To investigate why cells with this mutation gain a competitive advantage over normal cells, the team developed a mouse model carrying the Dnmt3a mutation.
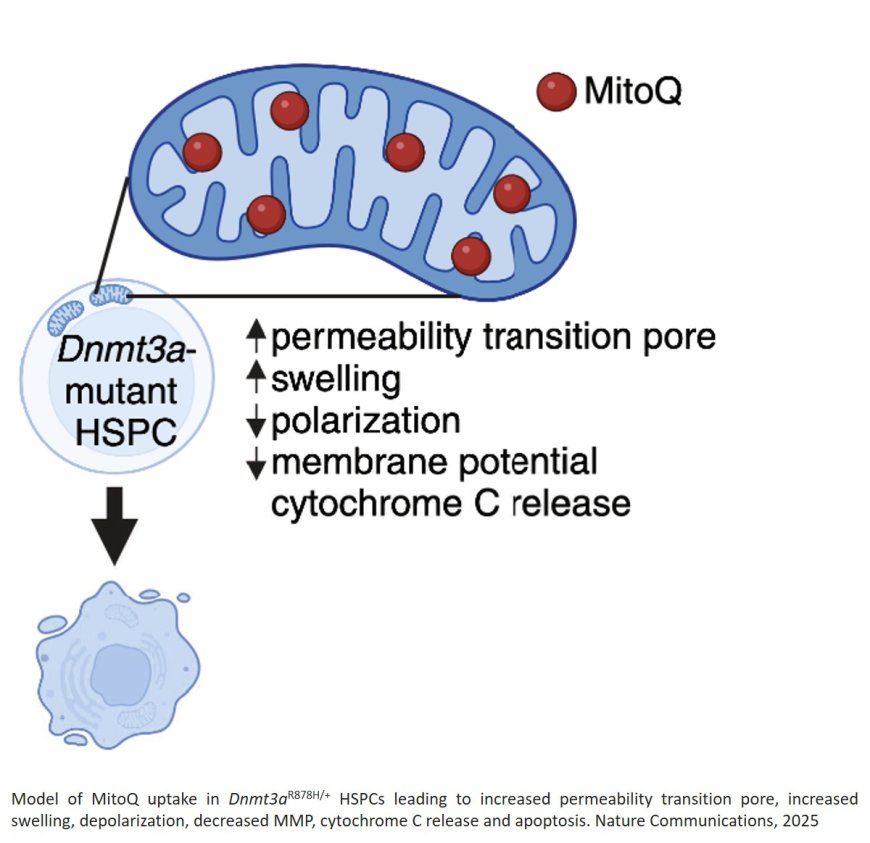
In this new study, the team discovered that in middle-aged mice, the mutated stem cells had double the energy-producing capacity of normal cells. The mutated stem cells also contained turbocharged mitochondria, which gave the cells a strong competitive growth advantage.
“This was really unexpected,” the author said. “This gene was not previously known to impact metabolism or mitochondria.”
The researchers realized that because the stem cells with Dnmt3a mutations relied so heavily on their overactive mitochondria to support their growth, the mitochondria might be an Achilles heel for the mutated cells. In isolated stem cells and mice with Dnmt3a mutations, the team tested the effect of MitoQ and d-TPP – molecules which disrupt the normal function of mitochondria and prevent them from producing energy.
In a separate paper co-published in Nature, the authors report that metformin—a first-line treatment for type 2 diabetes—also diminishes the competitive advantage of stem cells carrying the Dnmt3a mutation.
In mice with Dnmt3a mutations and clonal hematopoiesis, the mitochondria-targeting drugs had drastic effects. Within a few days of treatment, about half of all mutant cells died and among remaining mutant cells, their energy production dropped to normal levels. Normal cells – which don’t rely as heavily on the same metabolic pathway – were not impacted.
“Seeing this selective vulnerability where mutated cells were weakened, but normal stem cells are fine, was really exciting,” said the author.
The mitochondrial drugs worked not only in mice with clonal hematopoiesis, but in human blood stem cells engineered to have the DNMT3A gene mutation. The results suggest that the strategy could work in treating humans with the condition to prevent blood cancers and other age-related illnesses.
However, more work is needed to understand whether these drugs would be effective at targeting other mutations observed in clonal hematopoiesis and the effect of the drugs on cells.
https://www.nature.com/articles/s41586-025-08871-w
https://www.nature.com/articles/s41467-025-57238-2
https://sciencemission.com/Dnmt3a-mutant-clonal-hematopoiesis