Albumin redirects metabolism to turn harmless Candida albicans into dangerous pathogens
Candida albicans is part of the normal human microbiome, colonizing mucosal surfaces without causing harm. Under specific conditions, however, the fungus can become pathogenic – particularly in people with weakened immune systems. Scientists have long known that Candida albicans causes infections using well-described mechanisms, such as toxin production and hyphae formation to invade host tissues.
Now, an international research team has uncovered another tool with which the yeast can cause damage: “We discovered that Candida albicans can use an alternative pathogenicity strategy,” says the first author. “Even strains or mutants previously considered non-virulent in the lab became cytotoxic when albumin was present.”
The idea for the study arose from a puzzling observation: some clinical Candida isolates, although taken from infected patients, caused no noticeable damage in standard lab models. “That didn’t add up,” the author recalls. “We suspected that some important host-specific signal was missing from our test systems – and albumin was a likely candidate.”
Albumin is the most abundant protein in human blood serum. It plays various roles in transport, nutrient binding, and immune regulation. In carefully controlled infection models, the researchers found that albumin triggered a shift in fungal behavior: even previously non-harmful Candida strains began to grow more strongly, form biofilms, and release a cytotoxic lipid molecule called 13-HODE, which directly damages human cells.
“The fungus doesn’t necessarily need to grow long hyphae or produce great amounts of toxin in order to cause infection,” explains a co-first author. “Depending on the condition it’s facing, it will adapt – and it can take advantage of the host.”
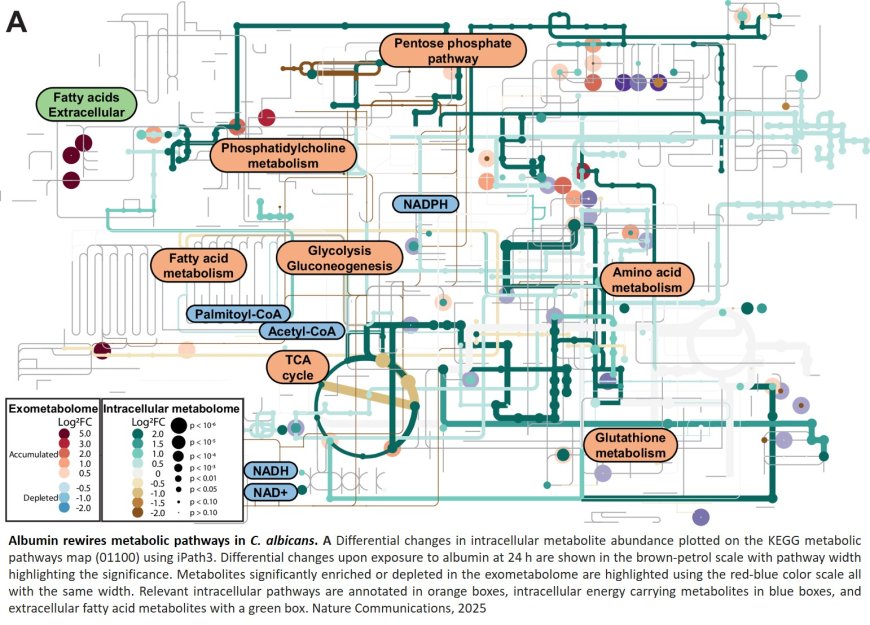
To uncover the mechanism leading to infection, the team used a combination of methods including microscopy, cell-based damage assays, transcriptomics, and metabolomics. They showed that albumin triggered a reprogramming of fungal metabolism, including lipid oxidation pathways that lead to the production of the toxic compound 13-HODE – which previously had not been associated with Candida albicans virulence.
The new findings highlight some important considerations for future fungal research. One is the need for physiologically relevant test systems that better reflect the human host environment. “Just providing essential nutrients in the lab is not enough,” says the author. “You need the right environmental cues. Otherwise, you might overlook strains that are actually dangerous in the human body.”
Another takeaway is the importance of working with clinical isolates that reflect the diversity of Candida strains in real-world infections. “If you want to study a vaginal infection for example, it makes sense to use a strain from that site,” says the co-first author. “Standard lab strains might not reflect what’s happening in a real infection.”
With this new understanding, Candida albicans emerges as an even more versatile organism that is capable of switching strategies depending on its environment – and even a single host protein like albumin can tip the balance toward disease. In the future, this knowledge may contribute to more realistic infection models and, in the long run, may help identify new targets for antifungal strategies.
https://www.nature.com/articles/s41467-025-61701-5
https://sciencemission.com/Host-albumin-redirects-Candida-albicans-metabolism