Chemical basis of aversive learning
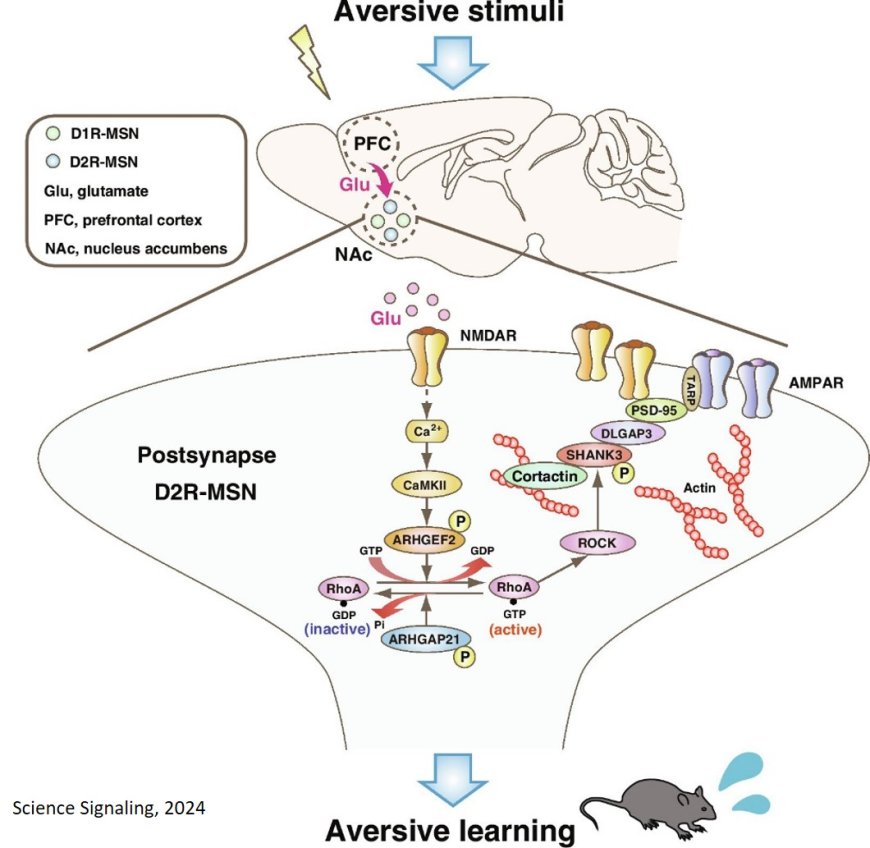
One of the things that makes brains so incredibly difficult to understand is their ability to adjust and adapt. Our learning experiences can set off complex signaling cascades that reshape neurons—and their synaptic connections—at the cellular level. For example, in mammals, scientists have established that activation of the N-methyl-D-aspartate receptor (NMDAR) and the ensuing calcium ion (Ca2+)-dependent signaling cascade is essential for postsynaptic remodeling and learning. As one might reasonably expect, problems with NMDAR have been linked to neuropsychiatric disorders, including epilepsy, schizophrenia, autism, and intellectual disabilities.
Understanding the functions of NMDAR is therefore key to understanding many neuropath ologies and developing treatments. Unfortunately, research in this field has been challenging because of the complexity of how NMDAR causes some of its downstream effects. More specifically, NMDAR activation triggers Ca2+-dependent kinases, such as CaMKII, that phosphorylate other target proteins. Phosphorylation is a chemical process that modifies proteins, acting as a molecular switch to turn various cellular functions on or off. Although these modifications are central to the functions of NMDAR, studying phosphorylation events and their roles in signaling pathways has proven difficult.
In a recent paper published in Science Signaling, they employed phosphoproteomics technique to shed light on how intracellular NMDAR signaling regulates synaptic plasticity and learning through phosphorylation events.
The abovevmentioned method is called kinase-oriented substrate screening (KIOSS), which uses affinity beads coated with domains that bind to phosphorylated serine and threonine residues. Using KIOSS, the researchers investigated phosphorylation events downstream of NMDAR in isolated mice brain slices of the nucleus accumbens (NAc), a key brain structure that is involved in emotional responses, motivation, and various forms of learning. Using an NMDAR agonist to induce a higher Ca2+ influx in neurons, they screened proteins phosphorylated by CaMKII.
The researchers found 194 proteins that fit this criterion, but they focused specifically on Rho GTPase regulators, which are known to play a crucial role for synaptic remodeling in learning. Interestingly, they observed that phosphorylation of a specific regulator called ARHGEF2 led to the activation of RhoA, which in turn activated Rho-kinase/ROCK, a protein necessary for proper neuronal development and structure.
The team decided to delve deeper into the effects of ROCK activation in this context, as the senior author explains: “ROCK inhibition impairs memory formation, including long-term spatial memory in the hippocampus and fear conditioning memory in the lateral amygdala. However, it remains unclear whether ROCK regulates aversive learning in the NAc. Thus, we used mice in a passive avoidance test, a fear-motivated test classically used to assess memory in laboratory animals, to examine whether ROCK regulates aversive learning.”
The researchers ultimately identified 221 candidate substrates of ROCK, and demonstrated via in vivo experiments that aversive stimuli activated the CaMKII–RhoA–ROCK pathway. Inhibiting this pathway chemically or through genetic modifications resulted in impaired aversive learning, cementing the results.
Worth noting, the researchers put all the data derived from this study in a publicly available database they had previously developed. “The phosphorylated proteins and their phosphorylation sites identified in this work are registered in our KANPHOS database,” mentions the senior author, “KANPHOS provides extensive data on different aspects of phosphorylation in the brain, including extracellular and intracellular signaling pathways, phosphorylated proteins, phosphorylation sites, responsible kinases, mouse models, and related behaviors and diseases. These data and the KANPHOS database itself form a useful resource for elucidating the mechanisms of NMDAR signaling in the brain.”
Taken together, the contributions of this study could be foundational to achieve a better understanding of many neuronal diseases. “The signaling pathways downstream of NMDAR involved in aversive learning we identified may provide insights into the mechanisms underlying the pathophysiology of psychiatric disorders such as schizophrenia,” concludes Prof. Kaibuchi. “The next step is to explore how these findings can contribute to the development of treatments aimed at ameliorating symptoms of these disorders,” he adds, optimistic about the future.
Let us wish them the best of luck in their endeavors so that the burden of these challenging diseases can be lessened and people suffering from them enjoy a better quality of life.