Spatial map of malaria infection in the liver
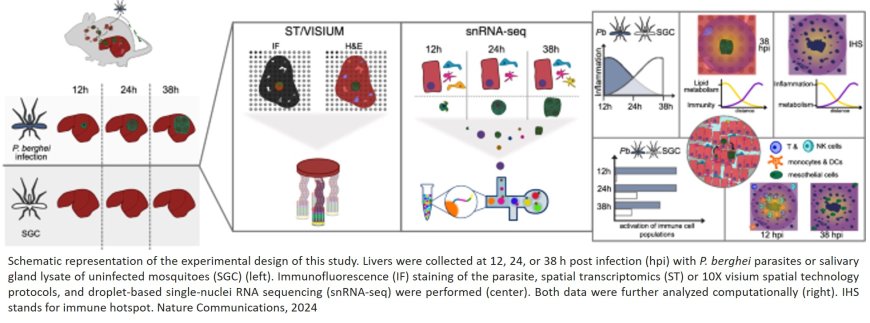
For the malaria parasite to reach the blood of its human host, it must first enter the liver, where only a small number of parasites differentiate and replicate for upwards of seven days, making it a bottleneck in the parasite’s lifecycle. This bottleneck makes the liver stage an optimal target for effective and long-lasting vaccines against the disease. Using Spatial Transcriptomics and single-cell RNA-sequencing technologies, researchers have for the first time managed to create a spatio-temporal map of malaria infection in the mouse liver. A study that was recently published in Nature Communications.
“The possibility to identify the exact location of differentially expressed genes across liver tissue sections in response to parasite infection has great potential to propel malaria research forward. It provides a whole new platform to study host-pathogen interactions in the true tissue context. This can eventually help researchers to identify novel targets for drug development and vaccine strategies for malaria, but also for a wider variety of pathogens that infect human tissues”, says the senior author.
The combination of Spatial Transcriptomics technology, and single-cell RNA-sequencing, allowed the researchers to chart the global gene expression of both the host and the parasite across Plasmodium berghei-infected mouse liver tissues, for the first time. The Spatial Transcriptomics method firstly enables researchers to generate an image of an entire tissue section, and secondly, through an array of thousands of spots, which all contain millions of barcoded probes, one can capture the global gene expression of small, micrometer scale, regions across the entire tissue section and ultimately link the expression profile back to specific locations in the imaging data. Further, merging the high-resolution single-cell data with the spatial data, enables the deconvolution of specific cell types in the tissue spots.
Malaria remains one of the deadliest diseases globally, with an estimated 230 million infections and over 600,000 fatalities every year, predominantly in young children. Symptoms only occur once the malaria causing parasite, Plasmodium spp, replicates in the blood. However, before the parasites reach the symptomatic blood stage, they first undergo an obligatory and clinically silent developmental stage in the liver. Every single parasite that enters the liver gives rise to tens of thousands of parasites, which are eventually released into the blood stream. There, they specifically infect the red blood cells and replicate into the billions.
The essential liver stage represents a major bottleneck in the parasite lifecycle as only very few parasites reach the organ and develop successfully. Therefore, this stage of the life cycle is considered an optimal target for the generation of effective and long-lasting vaccines against malaria. This goal is still not achieved despite the groundbreaking, recent release of two vaccine candidates, which have unfortunately shown low efficacy and a lack of long-lasting protection.
“In comparison to the symptomatic blood stage, the liver stage of the malaria lifecycle is highly understudied, and the development of an effective vaccine is impeded by the current lack of knowledge. The generation of a spatial map of Plasmodium liver infection in the true tissue context is a major advance in addressing this knowledge gap”, says the author.
The researchers discovered that the parasite causes changes in the gene expression of host cells in its proximity, in a time-dependent manner. During early liver-stage infection, they find pro-inflammatory gene programs in tissue locations in the proximity of parasite positions. In contrast, during the late stages of liver infection they find gene programs related to immune responses downregulated in parasite neighborhoods. Instead, they discovered an upregulation of gene programs related to fatty acid metabolism across host cells in the proximity of parasites at the late timepoint. Fatty acids are essential during the massive parasite replication that occurs at the end of the liver stage and are moreover reported to be involved in anti-inflammatory responses.
Therefore, the researchers suggest that the parasite hits two birds with one stone, as it likely evades host immune responses and supplies itself with essential nutrients by hijacking the regulation of host cell transcription of genes encoding fatty acid metabolism.
Moreover, they find that overall immune responses across liver tissues in non-infected control mice, injected with lysate of mosquito salivary glands (where Plasmodium parasites reside before being injected into the skin of a vertebrate host) are also upregulated but show a significant delay of more than 12 hours, providing important implications for future study design of similar studies.
The research group also discovered novel spatial components in the context of infection, which are characterized by a high cell density and express pro-inflammatory and immune activation gene signatures. They term these structures “inflammatory hotspots”, which are similar to structures previously observed in the context of viral infections in tissues, including the liver.
“Previous studies have established that some parasites will develop successfully in the liver to reach the blood while others are successfully targeted by the host immune system and eliminated. Therefore, we speculate that these structures may represent positions of successful parasite elimination”, says the lead author of the study.