ERK1/2 signaling targeting in cancer
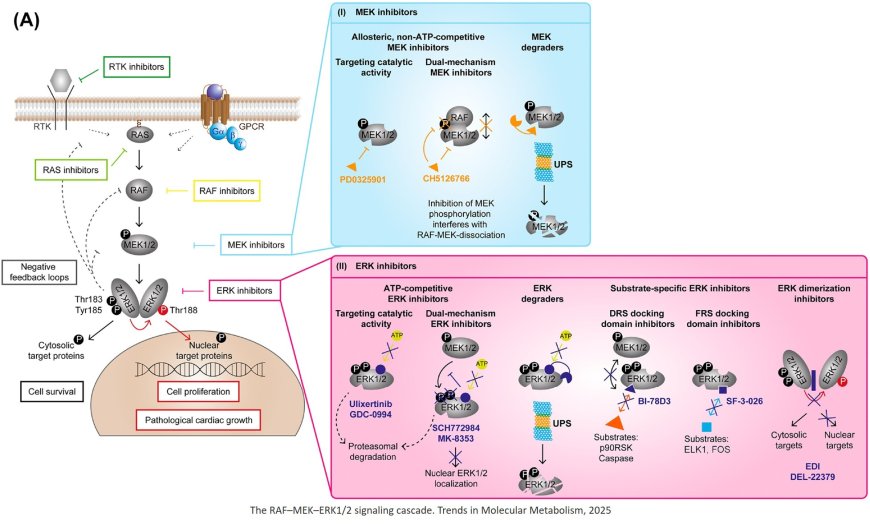
RAF inhibitors (RAFi) and MEK inhibitors (MEKi) and their combination have revolutionized cancer therapy. However, they often result only in short-term remission and limited tolerability often because of severe adverse effects such as cardiotoxicity.
ERK inhibitors (ERKi) such as ulixertinib, the first ERKi approved for the Expanded Access Program (EAP) by the FDA, provide an option for tumors resistant to RAFi/MEKi, highlighting their role in refractory settings.
Repurposing drugs targeting the ERK1/ 2 signaling cascade promises significant benefits in life-threatening RASopathy cases, and selumetinib and mirdametinib have been approved for neurofibromatosis type 1 with unresectable plexiform neurofibromas.
Current research focuses on the development of ERKi with higher selectivity for specific downstream targets with the aim of improving tolerability to prevent dose reductions or treatment discontinuations, to reduce acquired resistances, and ultimately to make these drugs suitable for non-malignant diseases.
https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(25)00189-3