Human Schwann cell lipid dysregulation and plasma membrane perturbation in Charcot–Marie–Tooth disease type 1A disease
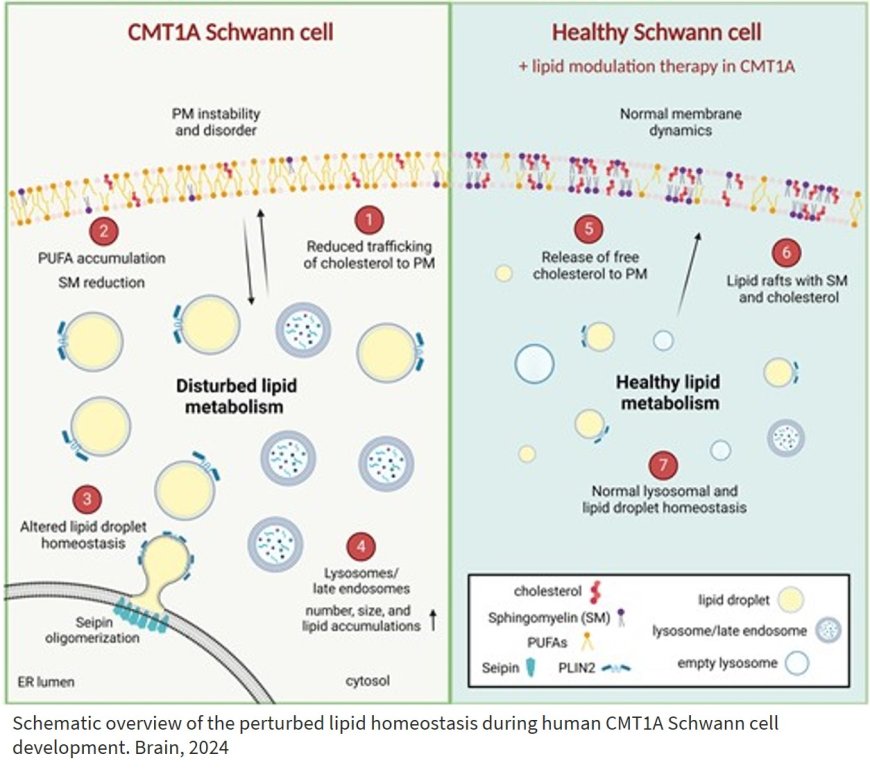
Chromosome 17 harboring the PMP22 gene is duplicated in Charcot–Marie–Tooth disease type 1A (CMT1A), the most common inherited peripheral neuropathy.
The researchers show that in human CMT1A Schwann precursor cells developed from iPSC’s, disordered plasma membrane (PM) and perturbation in PM lipid-mediated signalling, affecting the stability of the PM and cell differentiation.
PM instability arises from reduced cellular trafficking and incorporation of cholesterol; and excess accumulation of polyunsaturated fatty acids (PUFAs) and reduction of sphingomyelin (SM) in the plasma membrane (PM), thus, altering PM lipid composition.
In addition, the authors show that CMT1A Schwann cells displayed alterations in lipid droplet biogenesis, size and number during nutrient stress; and, likewise, the number and size of lysosomes and late endosomal lysosomes (LELs), and the number of lipid-loaded LELs was increased.
The authors demonstrate that targeting the lipid droplets results in release of free cholesterol and its incorporation into the PM; and stabilization of the PM and lipid raft dynamics.