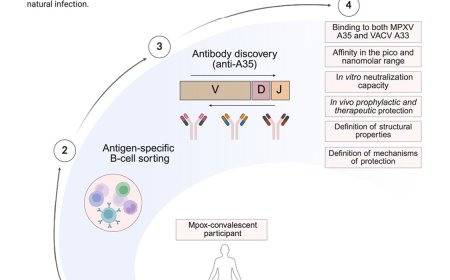
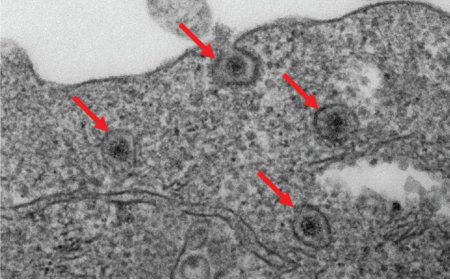
Inhibiting bacterial gene translation using siRNA
Multidrug-resistant bacteria pose a major threat to human health. Manipulation of bacterial genes at the transcriptional level is a potential strategy to fight antibiotic-resistant bacterial infections by silencing their resistance genes. However, siRNAs have not been applied to regulate bacterial genes due to the lack of RNAi regulatory machinery, i.e., RNA-induced silencing complexes (RISCs), in bacteria. In addition, efficient methods for delivering siRNAs to bacteria in vivo are not currently available. In this study, the authors demonstrated that exosomes can serve as delivery vehicles to introduce AGO2-loaded siRNA into the cytoplasm of bacteria, and in turn down-regulated gene expression of the mRNA that shares sequence complementarity to the siRNA.
The scientific significance of these findings is highlighted below:
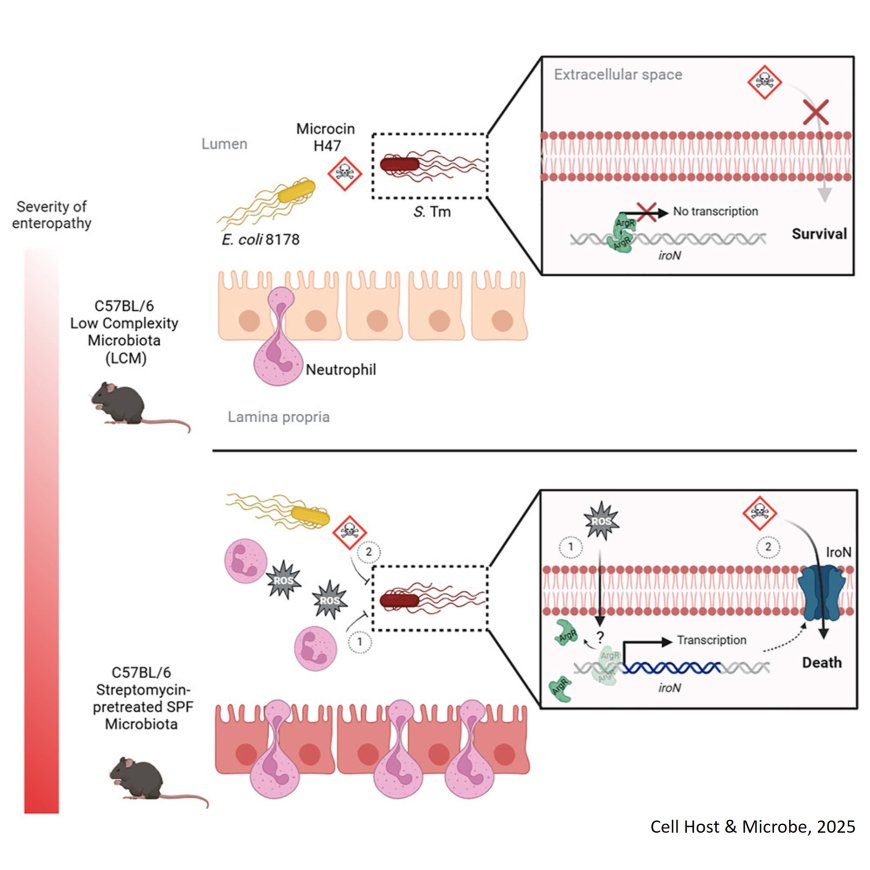
Despite the fact that the siRNA machinery is absent in prokaryote, this study demonstrates that exosomal siRNAs can be efficiently delivered into bacteria and silence target bacterial genes. The exosomal Argonaute 2 (AGO2) protein forms a complex with siRNAs, which is essential for siRNA-mediated gene silencing in bacteria. Even though the delivered siRNA was designed for possessing full sequence complementarity to the bacterial mRNA, downregulation of the target protein was achieved at the translational level rather than on the level of mRNA stability (as would be expected in mammalian systems).
Exosomal siMecA (an siRNA designed to target the mecA gene) can downregulate the mecA gene which encodes the penicillin-binding protein 2a (PBP2a), a protein at the heart of the drug-resistance phenotype in MRSA. Both in vitro and in vivo (using MRSA-infected mice) data demonstrated that by reducing PBP2a levels by exosome-delivered siRNA-AGO2 complex, MRSA can be converted into methicillin-sensitive bacteria.
To induce exosome production in vivo, the authors demonstrated that by intravenous injection of a plasmid encoding all relevant genes for the production of a particular siRNA into mice, the mouse liver was producing siRNA-AGO2 loaded exosomes (siMecA-Exos) efficiently targeting MRSA. These findings have huge potential clinical relevance since it might be possible to use this approach to also target multi-drug resistant bacterial infections in the human system.
In addition, exosome-mediated RNAi may function with imperfectly matched sequences, so exosomal miRNAs, which have multiple bacterial targets with imperfect base paring, may regulate bacterial genes in the same way as exosomal siRNAs. It is intriguing to consider the possibility that mammalian hosts employ exosomes to transport molecules with biological functions to the mammalian microbiome for interspecies communication and regulation under physiological conditions.
https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(25)00052-6
https://sciencemission.com/intestinal-inflammation-Salmonella--E-coli