Mitochondrial ketone body oxidation impaired in diabetes & liver disease
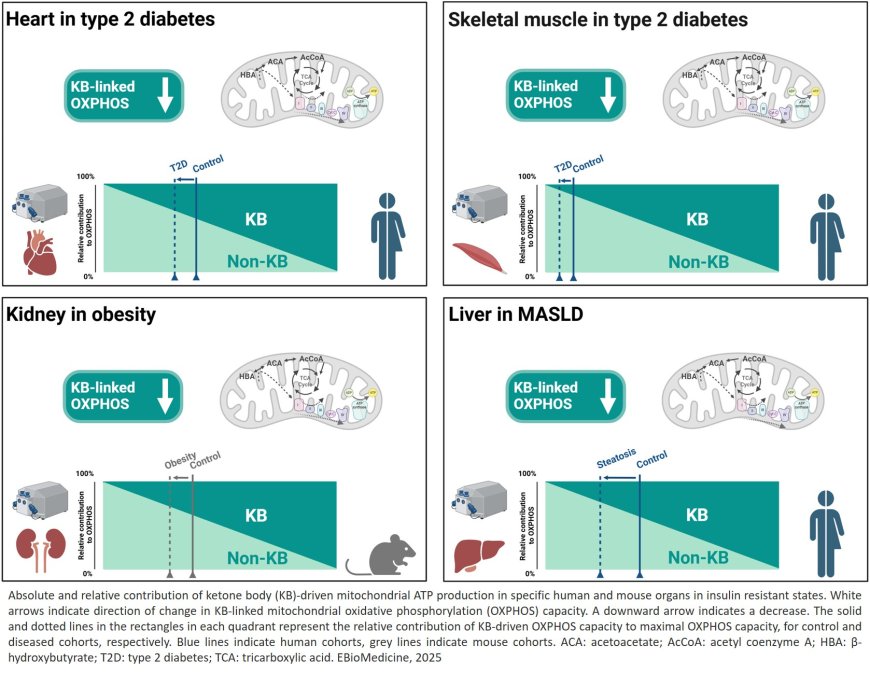
The liver plays a central role in storing and supplying energy to the body. In type 2 diabetes and metabolic dysfunction-associated steatotic liver disease (MASLD, commonly referred to as fatty liver disease), mitochondria—the cell’s power plants—cannot switch efficiently between fuel sources.
Scientists for the first time, how well mitochondria in heart, muscle, liver, and kidney can use the breakdown products of fats – ketone bodies – to produce energy. Ketone body levels can be increased through fasting, exercise, or low-carbohydrate diets.
The study shows that energy generation via ketone body metabolism is reduced in insulin resistance such as in type 2 diabetes and MASLD, highlighting a potential new target for improving energy metabolism in diabetes. Our bodies rely on the ability to switch between different energy sources depending on nutrient availability. Ketone bodies are small molecules made by the liver from fatty acids when glucose is scarce. They provide an alternative fuel for the heart, skeletal muscle, the kidney, and several other organs. An increased ketone level can support energy production in healthy individuals, but their benefit in this context depends on the mitochondria’s ability to utilize them.
“Ketone bodies are more than just an alternative fuel in specific conditions — they serve as important fuels for all domains of life to produce energy. In our study, we investigated if mitochondria in people with diabetes or fatty liver disease can still use them effectively,” says the senior author.
The researchers examined numerous tissue samples from obese people with and without type 2 diabetes or with and without MASLD. Using a novel approach based on a technique called high-resolution respirometry, the researchers were able, for the first time, to directly measure mitochondrial energy production from ketone bodies.
“Previous studies only looked at ketone body levels in the blood or organs, but our novel approach reveals the actual ketone body-driven energy output by mitochondria in the context of their cellular environment, offering a more representative view of metabolic alterations – even though it can only capture metabolism ex-vivo, not within the living organism”, explains the senior author.
The results were clear: in all the insulin-resistant states investigated, mitochondria produce less energy from ketone bodies. Compared to the respective control groups, heart and skeletal muscle cells of overweight individuals with type 2 diabetes and liver cells of overweight individuals with MASLD showed poorer utilization of ketone bodies for energy production.
“Interestingly, this defect was greater than the overall decline in mitochondrial function, suggesting that ketone body metabolism is particularly vulnerable in insulin resistance,” says the lead author.
These findings may have direct implications for people with diabetes, suggesting that simply increasing ketone body levels may not be sufficient to improve energy production, if mitochondria cannot use them efficaciously. Future treatments should be aimed at improving mitochondrial ketone body utilization and restore metabolic flexibility. Follow-up studies of the study group will further explore the mechanisms behind this altered ketone body metabolism and identify possible therapies.
https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(25)00451-7/fulltext