Phosphorylation by JNK switches BRD4 functions
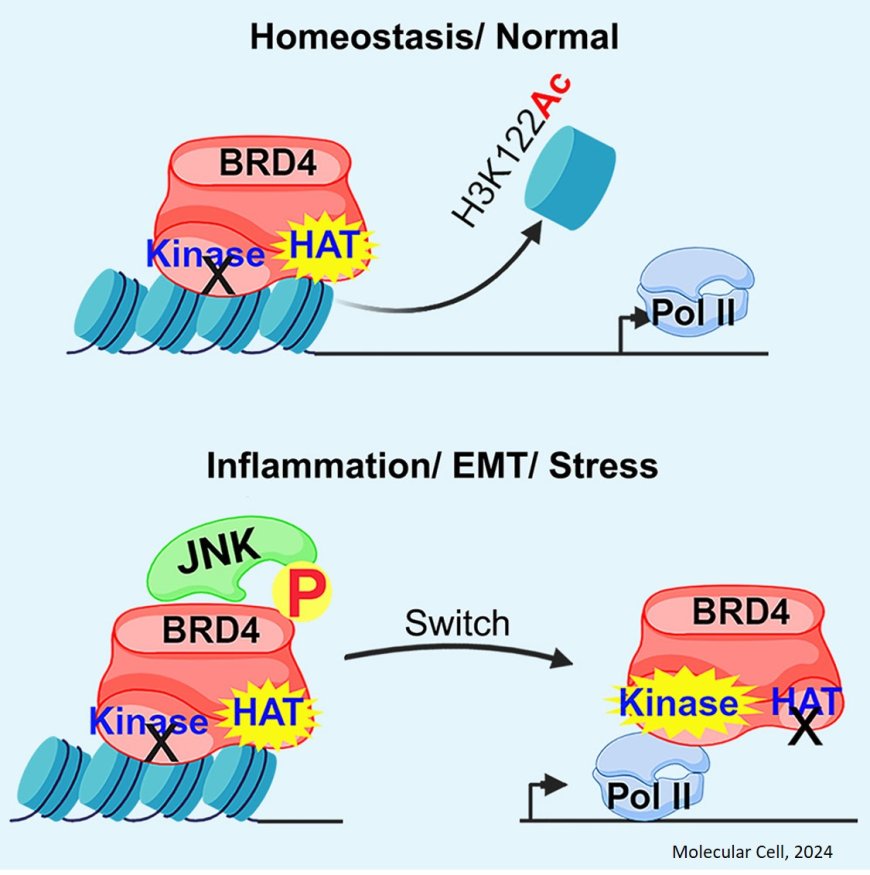
BRD4 is a nuclear protein that is functionally pleiotropic, alternatively regulating chromatin structure and transcription. chromatin-bound BRD4 regulates remodeling through its histone acetyltransferase (HAT) activity, while promoter-associated BRD4 regulates transcription through its kinase activity.
The researchers report that JNK phosphorylates BRD4, triggering a switch from chromatin modifier to transcription regulator, which promotes immune and inflammatory gene expression.
Mechanistically, JNK phosphorylates BRD4 at Thr1186 and Thr1212 triggers its transient release from chromatin, disrupting its HAT activity and potentiating its kinase activity. Released BRD4 directly interacts with and phosphorylates RNA Pol II, PTEFb, and c-Myc, thereby promoting transcription of target genes involved in immune and inflammatory responses. This functional switching also induces CD8 expression in thymocytes and epithelial-to-mesenchymal transition (EMT) in prostate cancer cells.
Therapeutic targeting of BRD4 will need to consider its functional switching.
https://www.cell.com/molecular-cell/fulltext/S1097-2765(24)00821-9
https://sciencemission.com/Phosphorylation-by-JNK-switches-BRD4-functions