Physiological role of 5-formylcytosine (5fC) epigenetic marker in the embryonic development
A research team has discovered that a DNA modification called 5-formylcytosine (5fC) functions as an activating epigenetic switch that kick-starts genes in early embryonic development. This finding proves for the first time that vertebrates have more than one type of epigenetic DNA mark and sheds new light on how genes are regulated in the earliest stages of development. Their findings were published in the journal Cell.
Our bodies are composed of trillions of cells, all working together to form a functional organism. Yet each of us started off as just a single fertilized egg cell. To become a whole human being, this single cell must multiply rapidly, forming all the correct organs in the right places. This process of development depends on thousands of genes being activated at exactly the right time and place. The activation/deactivation of genes is controlled by so-called epigenetic modifications, i.e., chemical groups attached to DNA and its associated proteins that act like traffic lights to switch genes on or off.
For decades, scientists thought that vertebrates had only one type of epigenetic modification on DNA called cytosine methylation, which is associated with gene silencing. Ten years ago, three more chemical modifications were discovered in vertebrate DNA, but as they were only present in very small amounts scientists were uncertain if they were functional epigenetic marks.
Now, a research team have shown for the first time that one of these modifications, 5-formylcytosine, is involved in activating genes in early development. The discovery is significant because it proves that vertebrates have more than one type of epigenetic DNA mark and uncovers a new, previously unknown mechanism of epigenetic gene regulation.
"These findings are a real breakthrough in epigenetics because 5fC is only the second proven epigenetic DNA modification besides methylcytosine," said the senior author.
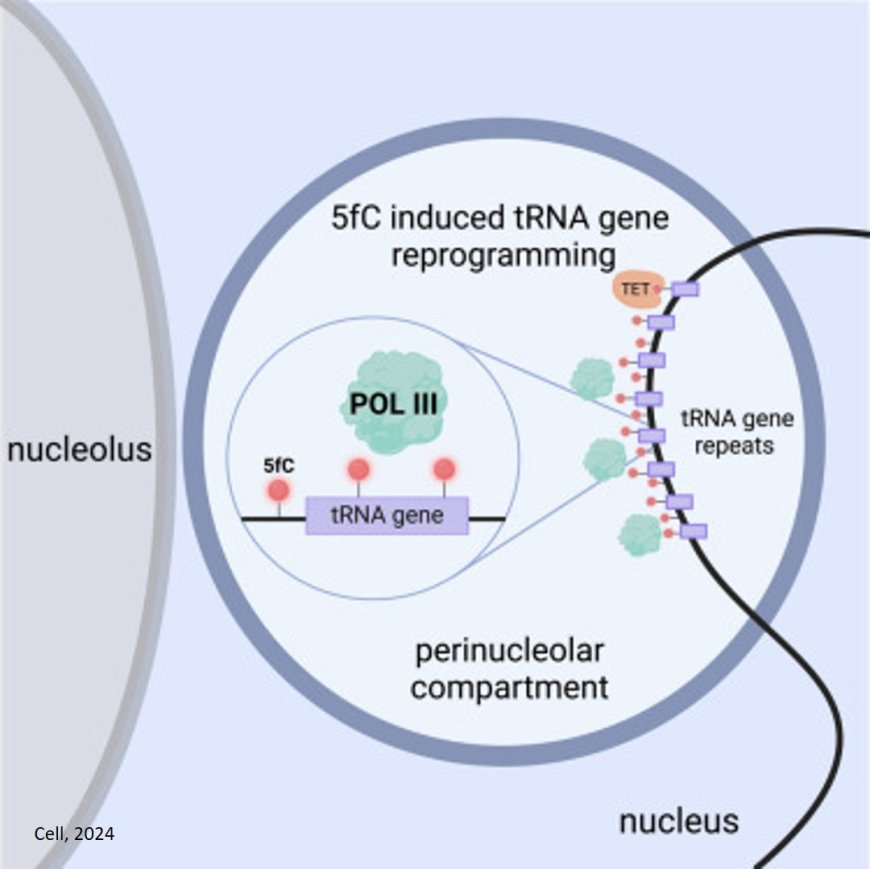
In their study, the scientists looked at 5fC in frog embryos. Using microscopy and chromatography, they discovered that 5fC increases dramatically at the very start of development during a key step called zygotic activation when many genes become switched on. As the first author of the study, explained: "The observation of 5fC in microscopically visible tiny dots, or chromocenters, was exciting. Based on them, we suspected that 5fC must do something important in early embryonic development."
To prove that 5fC is an activating epigenetic mark, the scientists genetically manipulated enzymes in the embryo to increase or decrease the amount of 5fC on the DNA. Increasing 5fC resulted in increased gene expression while decreasing 5fC reduced gene expression, indicating that it was indeed the presence of 5fC on the DNA that activates genes. Finally, the scientists also observed 5fC chromocenters in mouse embryos during zygotic gene activation. This suggested that 5fC likely acts as an activating epigenetic mark in both mammals and frogs.
The revelation that 5fC is an activating epigenetic regulator on DNA raises many questions as to how exactly it acts and what its role is beyond early zygotic genome activation. In particular, cancer cells can have very high amounts of 5fC. Additional studies on 5fC will be needed to answer these questions, which may ultimately help us to better understand how we develop and how gene regulation is disrupted in disease.