Role of transfer RNA fragments in cancer progression
This study was inspired by a serendipitous discovery in 2010, when the researchers found high levels of small RNA fragments derived from specific tRNAs, rather than the anticipated microRNAs in ovarian cancer samples. "At that time, the physiological roles of tRNA fragments were unknown. We felt compelled to investigate further to understand the role of these fragments in cancer," shares the senior author.
To investigate the role of a specific underexplored fragment, 5′-tRH-GlyGCC in cancer, the researchers employed a combination of cutting-edge molecular and biochemical techniques. Nanopore sequencing was used to analyze the transcriptome, while alternative splicing assays were performed to assess how the RNA fragment affected gene expression. Additionally, the interaction between 5′-tRH-GlyGCC and heterogeneous nuclear ribonucleoprotein (HNRNP) proteins, involved in splicing, was closely examined. Furthermore, the researchers conducted in vitro experiments to analyze cancer cell proliferation, and used in vivo xenograft mouse models to evaluate the therapeutic potential of targeting 5′-tRH-GlyGCC.
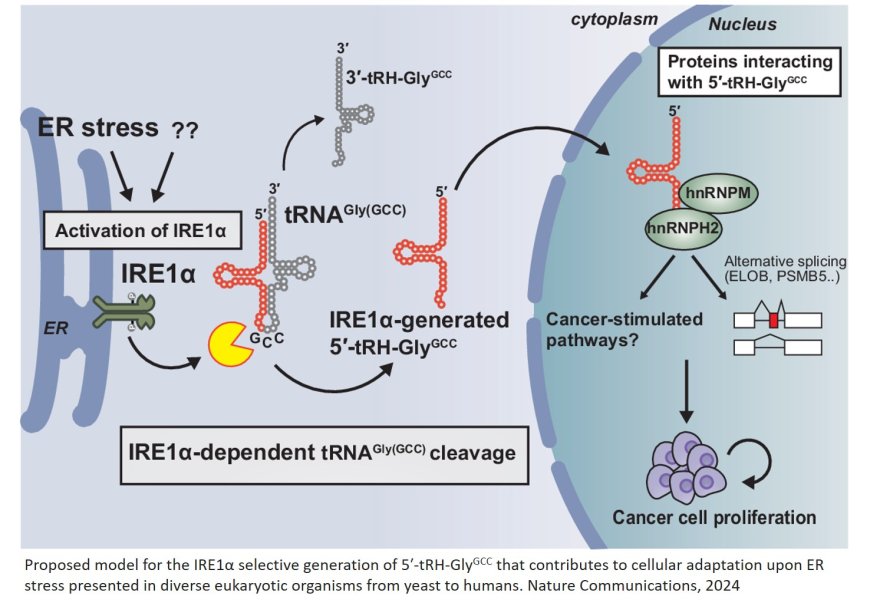
The researchers found that under endoplasmic reticulum stress, inositol-requiring enzyme 1α (IRE1α) splits tRNAGly(GCC) to produce 5′-tRH-GlyGCC. Furthermore, the results revealed that 5′-tRH-GlyGCC plays a critical role in alternative splicing and messenger RNA isoform regulation, influencing the expression of genes involved in cancer progression.
The study also found that this RNA fragment interacts with HNRNP proteins, which regulate splicing. In vitro experiments showed that modulating the levels of 5′-tRH-GlyGCC can significantly affect cancer cell proliferation. “Our study addresses a long-standing question in RNA and cancer biology. We have uncovered how specific tRNA fragments are produced and their critical roles in cellular stress and cancer, which opens novel opportunities for diagnostic and therapeutic applications,” explains the author.
In xenograft mouse models, suppressing this RNA fragment using antisense oligonucleotides (ASOs) led to tumor regression. These findings suggest that 5′-tRH-GlyGCC could serve as a biomarker for early-stage cancer detection, as its level can be easily detected in blood samples using RT-PCR, similar to coronavirus detection. Moreover, its role in tumor growth makes it a promising target for therapeutic interventions. The author explains, "Blocking these tRNA fragments led to tumor regression in mouse models. We are now exploring how to deliver ASOs into human cells, bringing us closer to clinical applications."
We hope this study paves the way for innovative approaches in precision medicine, leading to more effective diagnostics and personalized cancer therapies.