Structural basis of apoptosis induction
The activation and deactivation of apoptosis is a promising field of research in basic biomedical research. The team has now discovered a new switch:
"Many research teams worldwide are working on the exciting topic of apoptosis and its targeted control. The big advantage is that we are dealing with a highly efficient, evolutionarily developed regulatory mechanism. So, we don't have to invent something completely new, but can use the appropriate structural methods to learn from nature's optimized processes."
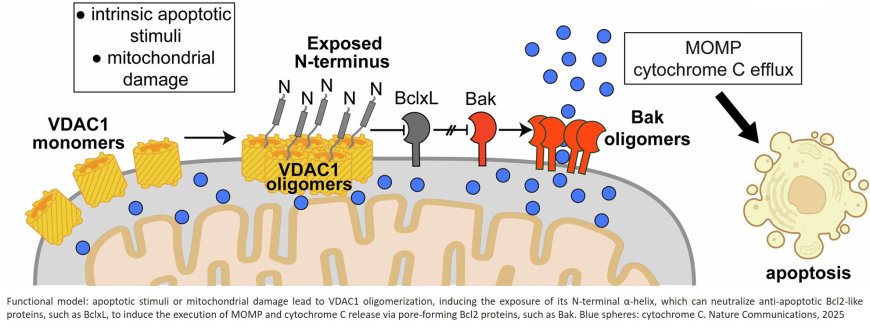
To prevent healthy cells from accidentally destroying themselves, the apoptosis system is very balanced. The researchers were able to show that the protein Bcl-xL, an apoptosis inhibitor that prevents overreactions, can be overridden by another protein called VDAC1 when necessary. The activation of this essential protein in the mitochondria, the powerhouses of the cell, is mainly triggered by increased cell stress, which can be an indication of abnormal cell development. VDAC1 then unfolds part of its structure, connects it to Bcl-xL, and thus deactivates the inhibitor.
This newly understood regulatory mechanism opens up possibilities for the search for active substances that could influence the behavior of VDAC1. In cancer therapy, for example, future drugs could specifically enhance activation and thus drive cancer cells to cell death. In neurodegenerative diseases such as Alzheimer's or Parkinson's, the opposite would be true. There, one could try to block the unwanted death of nerve cells. Deactivation of VDAC1 could also be helpful in certain heart diseases such as ischemia-reperfusion injury.
However, there is still a long way to go before these new findings can be applied clinically. The search for appropriate active substances can now begin. Whether it will be successful is completely open and will become clear after further experiments.