Structural basis of Nipah virus RNA synthesis
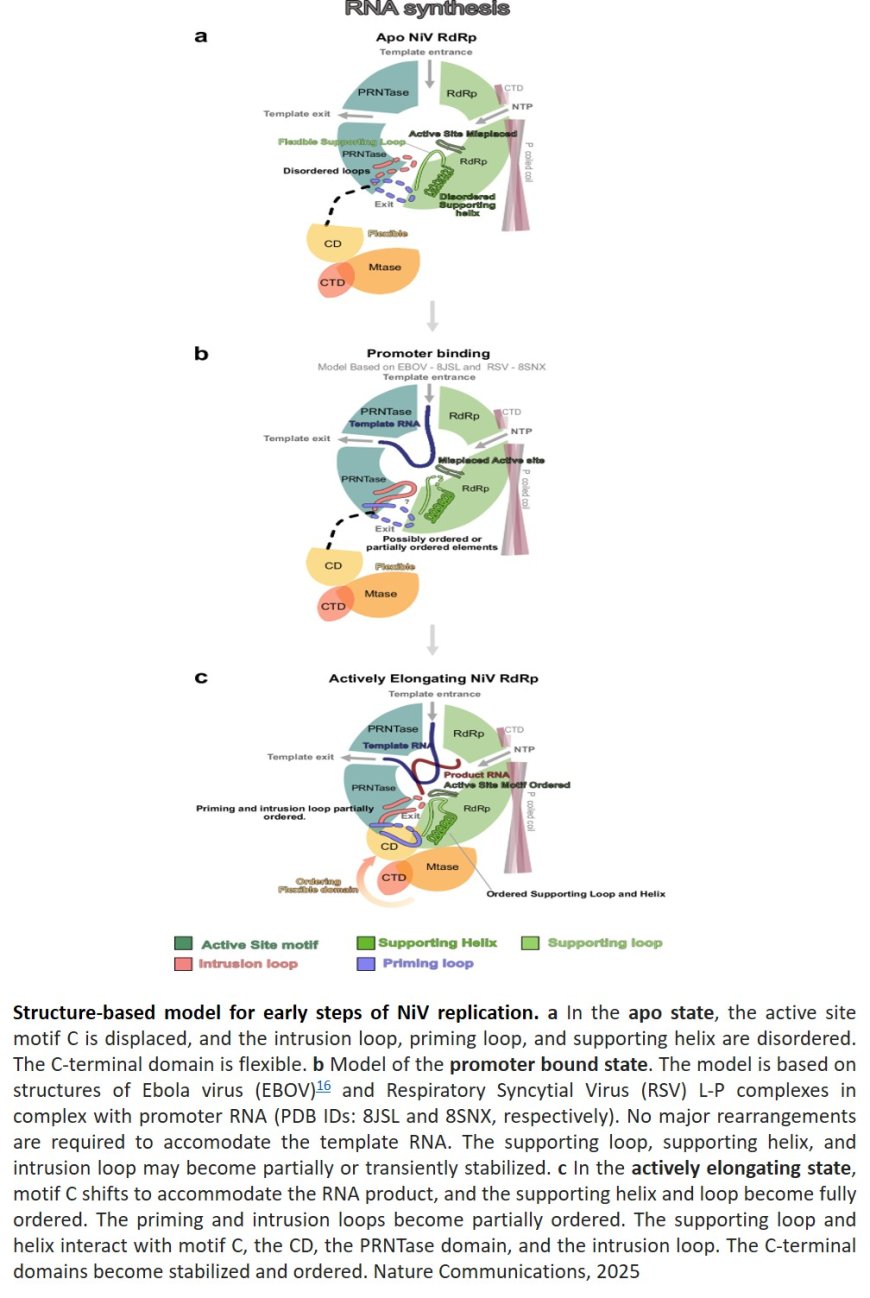
NiV is a non-segmented negative-strand RNA virus (nsNSV) and its RNA-dependent RNA polymerase (RdRp) complex, consisting of the L and P proteins, which is an attractive drug target.
The researchers describe cryo-EM structures of the NiV polymerase complex in the apo and in an early elongation state with RNA and incoming substrate bound.
They show that RNA binding leads to rearrangements of key elements in the RdRp core and to ordering of the flexible C-terminal domains of NiV L required for RNA capping.