E3 ubiquitin ligases in signaling, disease, and therapeutics
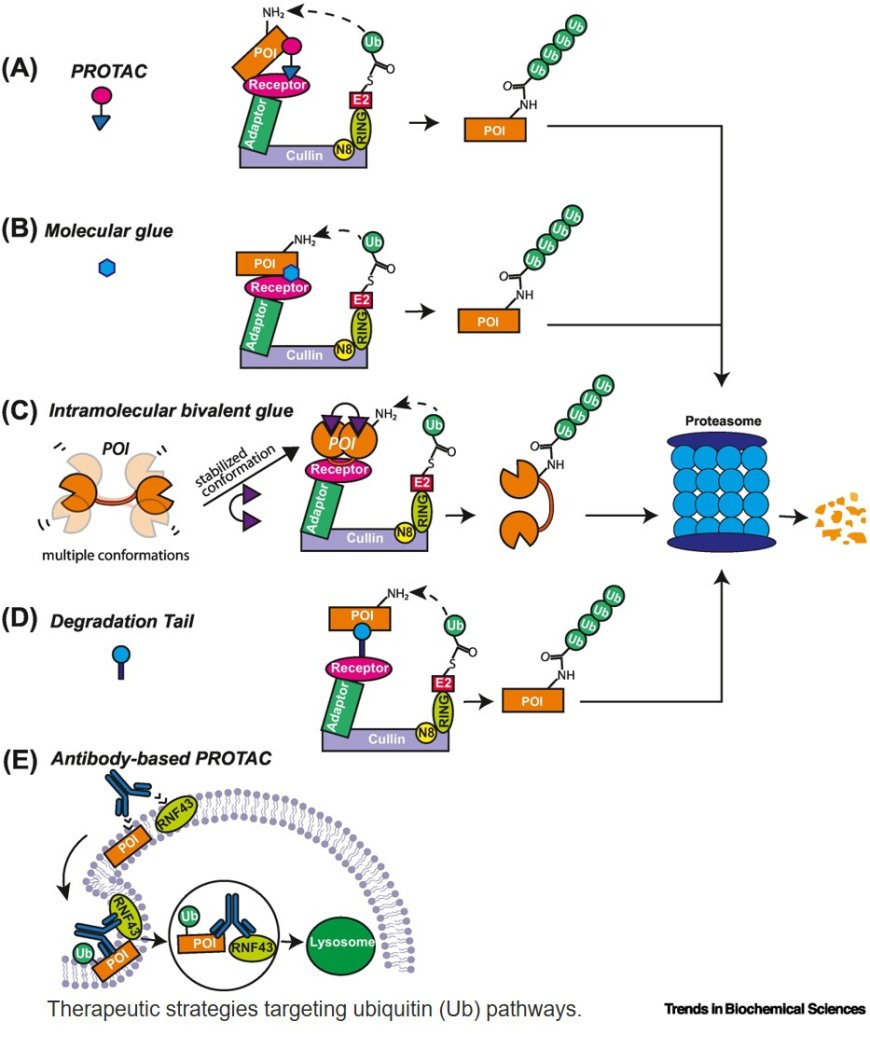
Recent structural and biochemical studies have revealed new classes of E3 ligases, including RING-Cys-Relay, RZ finger, and Cullin–RING ligase, RINGbetween-RING assemblies, expanding the mechanistic diversity of ubiquitin transfer.
Branched and mixed-linkage ubiquitin chains serve as complex regulatory signals, integrating cellular stress, signaling, and degradation pathways.
Advances in targeted protein degradation highlight the potential of E3-based strategies such as proteolysis-targeting chimeras and molecular glues to modulate previously undruggable proteins.
Emerging structural, chemoproteomic, and AI-guided tools are accelerating discovery of E3–substrate interactions and enabling rational degrader design.
https://www.cell.com/trends/biochemical-sciences/fulltext/S0968-0004(25)00186-0