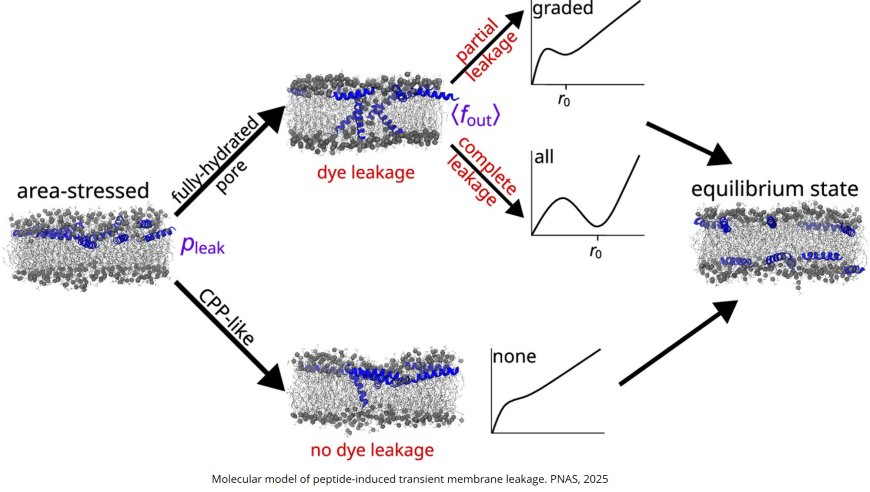
How antimicrobial peptides induce transient pores
New research into antimicrobial peptides, small chains of amino acids able to damage bacterial cells, shows why some peptides are more effective at doing that and also why some cells are more vulnerable.
The findings open the door to designing novel compounds for killing disease-causing organisms that have become resistant to antibiotics. These compounds would represent a major breakthrough against a pervasive global problem, said study co-leader.
“Antimicrobial resistance is a growing threat throughout the world,” said co-corresponding author. “In 2021, almost 5 million people died of antimicrobial resistance. Almost 40 million are predicted to die of it between 2025 and 2050.”
Found in all living things, peptides have a variety of forms and functions, including as hormones, neurotransmitters, and signaling molecules in cells. As antimicrobials, they help make up the first line of defense against bacterial infection, often by causing bacterial cells’ contents to leak out of the membrane, compromising cellular function.
The research focused on the specific states that give rise to membrane disruption. The study combined lab experiments with computational work shed light on the characteristics of the holes, known as pores, that antimicrobial peptides form in membranes. The pores form when the peptides that coat the outside of the cell start flipping across the membrane to balance out their numbers on each side.
“This information is very powerful since it provides the basis to explain why some peptides are more active than others and why some membranes are easier to target,” the author said. “We learned that the peptides that disrupt membranes more dramatically form pores in the membranes that are larger in size and number and stay open longer. It makes sense but the crucial novelty of our work is that we now have a mathematical equation that relates the effectiveness of membrane damage and pore characteristics. We can start applying it to identify properties required for optimal efficacy.”
The scientists also found that certain membranes carry defects that help peptides form that type of pore, insight that opens the door to designing peptides that specifically target bacterial cells based on their distinct membrane composition.
https://www.pnas.org/doi/10.1073/pnas.2510294122
https://sciencemission.com/poration-induced-by-antimicrobial-peptides