Antisense oligonucleotide therapeutic approach for Timothy syndrome
In a proof-of-concept study, researchers demonstrated the effectiveness of a potential new therapy for Timothy syndrome, an often life-threatening and rare genetic disorder that affects a wide range of bodily systems, leading to severe cardiac, neurological, and psychiatric symptoms as well as physical differences such as webbed fingers and toes.
The treatment restored typical cellular function in 3D structures created from cells of people with Timothy syndrome, known as organoids, which can mimic the function of cells in the body. These results could serve as the foundation for new treatment approaches for the disorder. The study, supported by the National Institutes of Health (NIH), appears in the journal Nature.
"Not only do these findings offer a potential road map to treat Timothy syndrome, but research into this condition also offers broader insights into other rare genetic conditions and mental disorders," said Joshua A. Gordon, M.D., Ph.D., director of the National Institute of Mental Health, part of NIH.
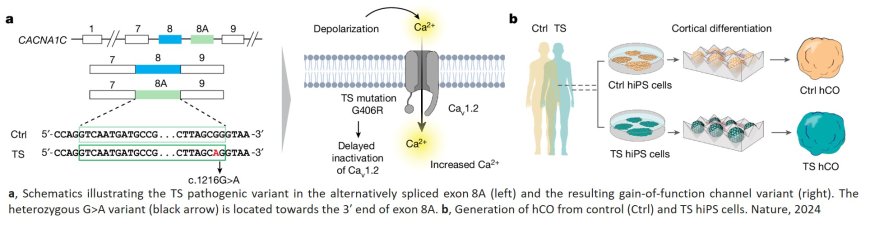
The researchers collected cells from three people with Timothy syndrome and three people without Timothy syndrome and examined a specific region of a gene known as CACNA1C that harbors a mutation that causes Timothy syndrome. They tested whether they could use small pieces of genetic material that bind to gene products and promote the production of a protein not carrying the mutation, known as antisense oligonucleotides (ASOs), to restore cellular deficits underlying the syndrome.
In the lab, researchers applied the ASOs to human brain tissue structures grown from human cells, known as organoids, and tissue structures formed through the integration of multiple cell types, known as assembloids. They also analyzed organoids transplanted into the brains of rats. All of the methods were created using cells from people with Timothy syndrome. Applying the ASOs restored normal functioning in the cells, and the therapy's effects were dose-dependent and lasted at least 90 days.
"Our study showed that we can correct cellular deficits associated with Timothy syndrome," said the author. "We are now actively working towards translating these findings into the clinic, bringing hope that one day we may have an effective treatment for this devastating neurodevelopmental disorder.
The genetic mutation that causes Timothy syndrome affects the exon 8A region of the CACNA1C gene. The gene contains instructions for controlling calcium channels—pores in the cell critical for cellular communication. The CACNA1C gene in humans also contains another region (exon 8) that controls calcium channels but is not impacted in Timothy syndrome type 1. The ASOs tested in this study decreased the use of the mutated exon 8A and increased reliance on the nonaffected exon 8, restoring normal calcium channel functioning.