Supramolecular anticoagulants with on-demand reversibility
Anticoagulant treatments are crucial for managing many conditions, such as heart disease, stroke and venous thrombosis. Current options, however, carry an inherent risk of serious bleeding due to trauma or unforeseen events. A research team has developed a new anticoagulant, designed to have an on-demand reversible activity, with a fast-acting ‘‘antidote’’. This approach could revolutionise the use of anticoagulants in surgery or other applications. The mechanism of activation and deactivation of the active principle could also be used in immunotherapy. These results are published in Nature Biotechnology.
Anticoagulant therapies are essential for managing many conditions, such as heart disease, stroke, and venous thrombosis. However, current treatment options, such as heparin and warfarin, have major drawbacks, including the need for regular monitoring of blood coagulation and the risk of serious bleeding in the event of overdose or trauma. Around 15% of emergency hospital visits for adverse drug effect are attributable to complication with anticoagulant treatments (an estimated 235 000 case/year in the US), emphasizing the importance of developing new, safer, and more effective therapeutic options.
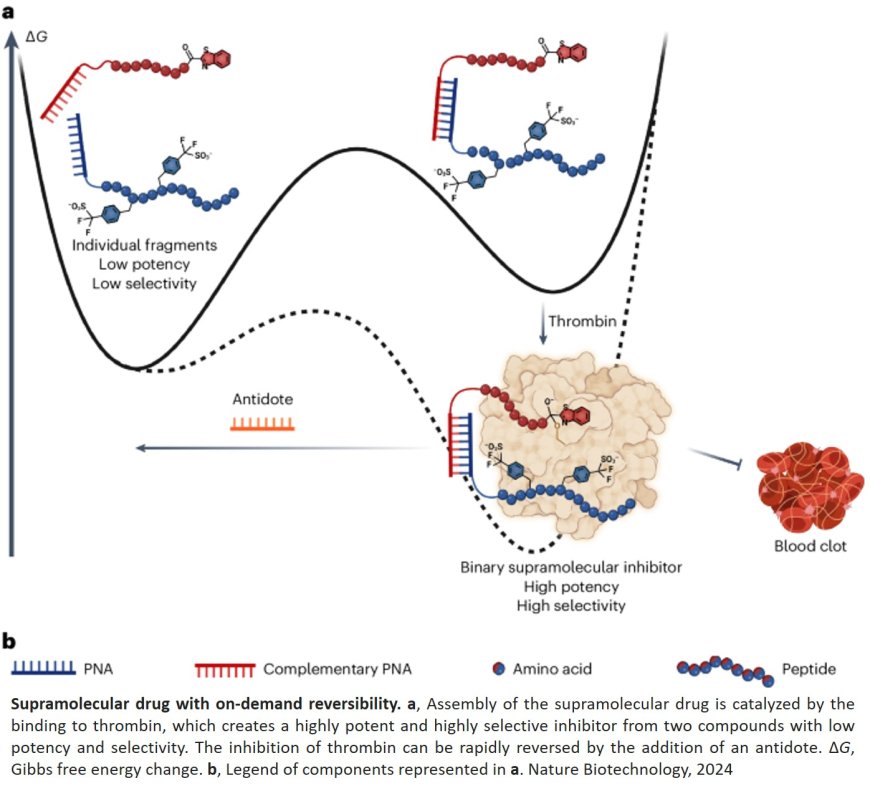
The group has recently developed a new anticoagulant active ingredient with an ‘‘antidote’’ to reverse its effect rapidly and specifically. This new active ingredient, presented in Nature Biotechnology, consists of two molecules targeting distinct sites of thrombin, a protein whose action is central for blood coagulation. After binding to thrombin, these two molecules combine to inhibit its activity, thereby reducing its coagulant effect. The antidote intervenes by dissociating these two molecules, thus neutralising the action of the active ingredient.
‘‘This breakthrough goes beyond the development of a new anticoagulant and its associated antidote. The supramolecular approach proposed is remarkably flexible and can be easily adapted to other therapeutic targets. It is particularly promising in the field of immunotherapy,’’ explains the senior author.
This new anticoagulant could offer a more reliable and easier-to-use option for surgical procedures. Heparin, commonly used in this field, is a mixture of polymers of different lengths extracted from pig intestine. The result is a highly variable action, requiring coagulation tests during surgery. The new synthetic anticoagulant developed by UNIGE could help solve the problems of purity and availability associated with heparin.
One of the breakthroughs in this work lies in the use of peptide nucleic acid (PNA) to link the two molecules that bind to thrombin. Two strands of PNA can come together via relatively weak bonds that are easy to break. The research team has shown that by introducing correctly designated strands of free PNA, it is possible to dissociate the two thrombin-binding molecules associated with each other. The free PNA strand thus deactivates the drug’s action. This is a major innovation in the field.
Beyond the problem of anticoagulation, this supramolecular concept of activating/deactivating the active principle could be of major interest in the field of immunotherapy, particularly for CAR-T therapies. Although CAR-T therapies are major advances in the treatment of certain cancers in recent years, their use is associated with a significant risk of immune system overreaction (cytokine storm), which can be life-threatening. The ability to rapidly deactivate a treatment with an accessible antidote could therefore represent a crucial advance in improving the safety and efficacy of these therapies.