Early developmental spinal cord extracellular matrix can promote neural regeneration
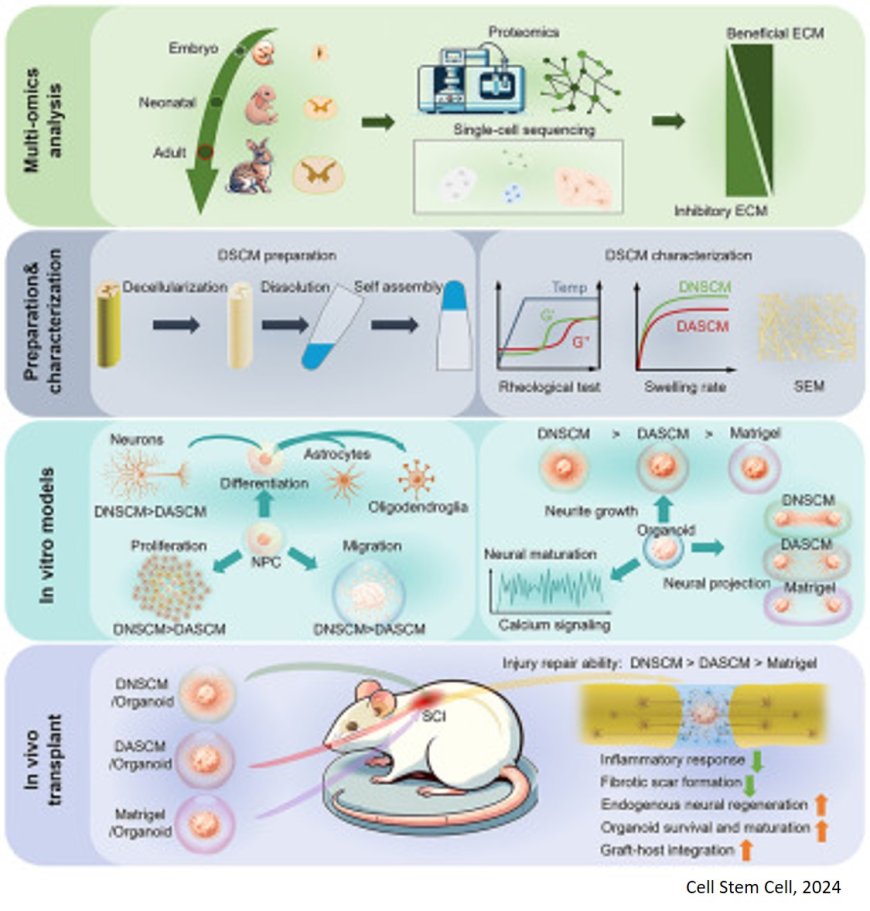
In a study published in Cell Stem Cell, researchers have demonstrated the remarkable role of early developmental spinal cord extracellular matrix (ECM) in promoting neuronal axon growth and functional maturation, as well as enhancing the therapeutic efficacy of neural progenitor cells (NPCs) and spinal cord organoids in rat spinal cord injury (SCI).
For adult mammals, SCI is a devastating blow due to the presence of multiple factors that inhibit regeneration within spinal cord tissues along with the weak regenerative capacity of neurons, ultimately leading to functional loss after injury. Scientists have long sought to understand the biological mechanisms underlying the inadequate regenerative capacity of adult spinal cord tissue. However, achieving satisfactory repair outcomes remains challenging, making nerve regeneration after SCI a global puzzle.
In contrast to adult tissues, embryonic and neonatal spinal cord tissues exhibit robust regenerative capabilities after injury. A detailed analysis of the differences in the tissue microenvironment during different developmental stages of the spinal cord is key to elucidate spinal cord regeneration mechanisms.
In this study, using multi-omics analysis, the researchers discovered that the embryonic and neonatal spinal cords harbor a higher concentration of neurodevelopment-related ECM molecules while exhibiting lower levels of inhibitory ECM molecules compared to the adult spinal cord. Leveraging decellularization technology, they isolated ECM components from spinal cords at the embryonic, neonatal, and adult stages, effectively preserving the distinct ECM compositions observed at these developmental points.
Their investigations revealed that neonatal spinal cord ECM significantly enhanced the proliferation, migration, and neuronal differentiation of NPCs, as well as promoted axon growth and functional maturation in neurons when compared to adult spinal cord ECM.
Specifically, the researchers successfully engineered spinal cord organoids using spinal cord ECM and found that neonatal spinal cord ECM facilitated superior axon extension and functional maturation within the organoids. In addition, their work demonstrated that tenascin (TNC) and pleiotrophin (PTN) proteins were key factors driving axon extension within organoids supported by neonatal spinal cord ECM.
Subsequently, NPCs and spinal organoids were integrated into the spinal cord ECM for SCI repair. Results showed that neonatal ECM weakened the inhibitory microenvironment after SCI and promoted enhanced migration and integration of NPCs into the host spinal cord tissue.
Furthermore, neonatal spinal cord ECM facilitated prolonged survival and functional maturation of organoids following transplantation, thereby enhancing the integration between organoids and regenerated nerve fibers within the host environment, resulting in improved efficiency of nerve signaling.
This study highlights the critical influence of early developmental spinal cord ECM in orchestrating spinal cord regeneration processes. By elucidating these mechanisms, the research provides both a conceptual framework and technical basis for utilizing early developmental spinal cord ECM in SCI repair strategies.