|
Researchers have refined a powerful DNA sequencing tool that can uncover hidden mutations that occur naturally in our bodies as we age. In the largest study to date, they have used the tool to provide insights into the earliest steps of cancer development and the role of mutations in healthy tissue.
The new study was published in the journal, Nature. The researchers introduce an improved version of nanorate sequencing (NanoSeq) – an ultra-accurate DNA sequencing technique.
By applying targeted NanoSeq to cheek swabs and blood samples from more than 1,000 volunteers, the team uncovered a rich landscape of mutations in healthy tissues, giving the most detailed picture so far of how tissues mutate over time.
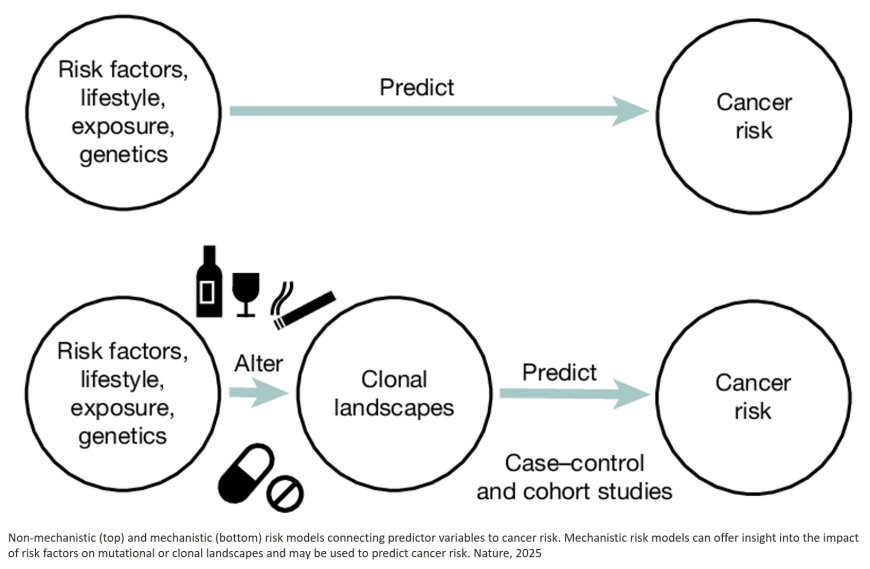
As people age, their cells naturally acquire DNA mutations which are known as somatic mutations. Most are harmless, but some can provide a growth advantage, leading to ‘clones’ of cells that carry the same mutations. As they multiply, some patches of clones have the potential to become the earliest stage in cancer development, but they may also contribute to ageing and other diseases.
Whilst detecting mutations in tumors is straightforward, historically, locating rarer mutations in normal tissues has been extremely challenging. This is because most sequencing methods do not have the accuracy to distinguish real mutations from errors in biopsies composed of thousands of clones, like most non-invasive biopsies.
To overcome this, researchers refined NanoSeq,1 so that it can precisely measure mutation rates, identify mutation patterns, and detect key driver mutations in any tissue.
In this new study and for the first time, the researchers used targeted NanoSeq to analyse non-invasive human samples – cheek swabs – from 1,042 participants in the TwinsUK cohort, alongside 371 blood samples. The volunteers ranged in age from 21 to 91, and included smokers and non-smokers, people with different histories of alcohol consumption, and varied lifestyles and exposures to cancer.
The researchers discovered over 340,000 mutations in cheek cells, including over 62,000 in genes known to drive cancer. They identified 49 genes under positive selection, which means they have mutations that give cells a growth advantage, including many well-known cancer genes such as TP53.
The study also revealed clear mutational signatures – patterns of mutations in the genome – linked to ageing, tobacco smoking and alcohol consumption. For example, smoking was associated with more mutations in the NOTCH1 gene and more growth of mutant clones, while heavy drinking left a distinctive pattern of DNA changes. Importantly, most mutated clones in normal tissue were found to be very small and did not continuously grow over time. This suggests that while mutations are common, most mutated cells are prevented from expanding and progressing to cancer.
By combining sampling on a large scale with an improved, highly accurate sequencing tool, this research provides the most detailed picture yet of how normal tissues mutate and evolve over time. The findings open the door to using NanoSeq to directly measure how lifestyle, environment and inherited factors influence DNA.
The improved version of NanoSeq is also being used more widely and is now the workhorse of other cancer and human genetics research.
Co-first author said: “We’re proud to present targeted NanoSeq, a new method that has completely transformed our ability to study somatic mutations in normal and diseased tissues. We’ve used NanoSeq to begin to understand the earliest steps in cancer development and uncover the role of somatic mutations in ageing and different diseases.”
Another co-first author said: “This is the largest study to date on how somatic mutations accumulate in a human tissue, as a result of ageing, smoking, alcohol, biological sex and other risk factors. Mutational landscapes could one day be used as measurable indicators of cancer risk, allowing earlier and more precise interventions.”
Senior author said: “With NanoSeq, we are able to measure the genetic consequences of certain lifestyle factors in normal tissues, meaning we can better understand why and how they cause cancer. We hope that this new ability to study somatic mutations in non-invasive tissue biopsies from healthy individuals becomes a useful tool for cancer prevention, by improving our ability to identify exposures in the population that could be mutagenic and carcinogenic, and by helping in the discovery of cancer preventive drugs.”
|