Intermittent fasting protects against liver inflammation and liver cancer
Fatty liver disease often leads to chronic liver inflammation and can even result in liver cancer. Scientists have now shown in mice that intermittent fasting on a 5:2 schedule can halt this development. The fasting regime reduces the development of liver cancer in mice with pre-existing liver inflammation.
The researchers identified two proteins in liver cells that are jointly responsible for the protective effect of fasting. An approved drug can partially mimic this effect.
The most common chronic liver condition is non-alcoholic fatty liver disease. It can have serious consequences: If left untreated, it can lead to liver inflammation (metabolic dysfunction-associated steatohepatitis, MASH), liver cirrhosis and even liver cancer.
Fatty liver disease is largely considered to be a direct consequence of obesity. It is not only people in Europe and the USA who have put on enormous amounts of weight in recent decades; obesity is also becoming increasingly widespread in emerging countries such as India and China. As a result, the number of cases of liver failure and liver cancer is rising sharply in the countries affected.
"The vicious circle of an unhealthy diet, obesity, liver inflammation and liver cancer is associated with major restrictions and suffering for those affected and also represents a considerable burden on healthcare systems," says the author. "We have therefore investigated whether simple dietary changes can specifically interrupt this fatal process."
Intermittent fasting has already been shown in several studies to be an effective means of reducing weight and alleviating certain metabolic disorders. The team has now tested in mice whether this approach can also protect the liver from fatty degeneration and chronic inflammation.
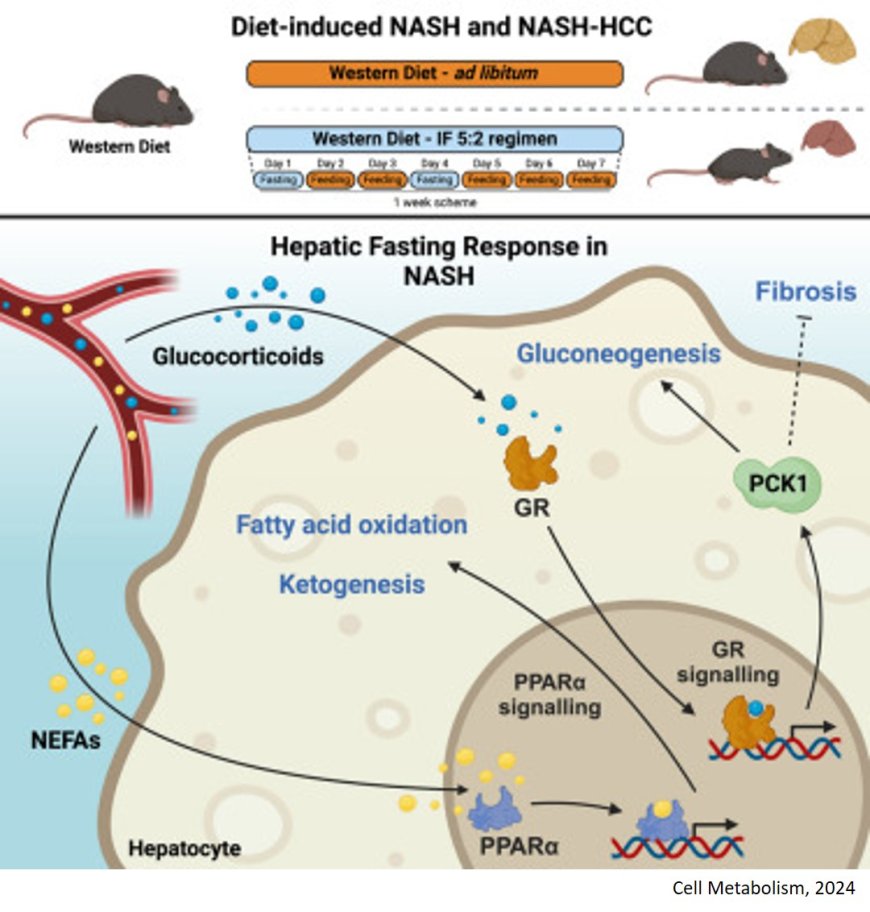
The animals were fed with a high-sugar and high-fat diet correspondind to the typical Western diet. One group of mice had constant access to the food. As expected, these animals gained weight and body fat and developed chronic liver inflammation.
The mice in the other group were given nothing to eat on two days a week (5:2 intermittent fasting, or 5:2 IF for short), but were allowed to eat as much as they wished on the other days. Despite the high-calorie diet, these animals did not put on weight, showed fewer signs of liver disease and had lower levels of biomarkers that indicate liver damage. In short, they were resistant to the development of MASH.
Interestingly, resistance to the development of a fatty liver was independent of the total calorie intake, as the animals immediately made up for the lost rations after the end of the fasting periods.
When experimenting with different variants of intermittent fasting, it was found that several parameters determine protection against liver inflammation: The number and duration of fasting cycles play a role, as does the start of the fasting phase. A 5:2 dietary pattern works better than 6:1; 24-hour fasting phases better than 12-hour ones. A particularly unhealthy diet requires more frequent dieting cycles.
The team now wanted to find out the molecular background of the response to fasting. To this end, the researchers compared protein composition, metabolic pathways and gene activity in the liver of fasting and non-fasting mice. Two main players responsible for the protective fasting response emerged: the transcription factor PPARα and the enzyme PCK1. The two molecular players work together to increase the breakdown of fatty acids and gluconeogenesis and inhibit the build-up of fats.
"The fasting cycles lead to profound metabolic changes, which together act as beneficial detoxification mechanisms and help to combat MASH," says the author, summarizing the molecular details.
The fact that these correlations are not just a mouse phenomenon was shown when tissue samples from MASH patients were examined: Here, too, the researchers found the same molecular pattern with reduced PPAR α and PCK1. Are PPAR α and PCK1 actually responsible for the beneficial effects of fasting? When both proteins were genetically switched off simultaneously in the liver cells of the mice, intermittent fasting was unable to prevent either chronic inflammation or fibrosis.
The drug pemafibrate mimics the effects of PPARα in the cell. Can the substance also mimic the protective effect of fasting? The researchers investigated this question in mice. Pemafibrate induced some of the favorable metabolic changes that were observed with 5:2 fasting. However, it was only able to partially mimic the protective effects of fasting. "This is hardly surprising, as we can only influence one of the two key players with pemafibrate. Unfortunately, a drug that mimics the effects of PCK1 is not yet available," explains the author.
While the team initially focused on the effects of intermittent fasting on the prevention of MASH, they then investigated whether the 5:2 diet could also alleviate existing chronic liver inflammation.