Role of fiber, genes and the gut microbiome in inflammatory bowel disease
Abdominal pain, diarrhea, weight loss—these and the other symptoms of inflammatory bowel disease (IBD) can be disruptive and debilitating. And while scientists have figured out that IBD has a genetic component, not everyone with a family history develops the disease. To date, the environmental triggers for Crohn’s disease and ulcerative colitis, known together as IBD, remain largely unknown.
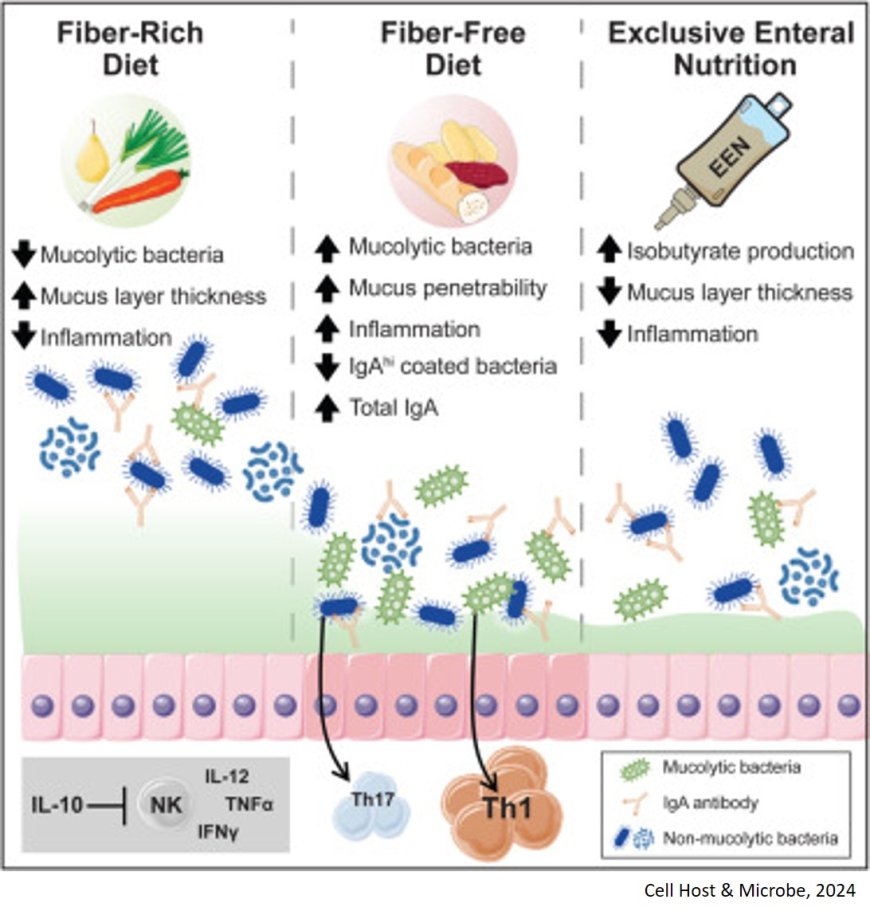
A new study finds a complex interplay between diet, genes, and the gut microbiota that could explain why IBD develops. The newest study builds on previous work that found that a low fiber diet led to a proliferation of mucin degrading bacteria—bacteria that thrive by eating the mucus lining of the intestine.
In some people, genetic loss of a cytokine—a protein that affects the immune system—known as interleukin-10 (IL-10), or its receptor leads to early onset of IBD in infants and children. The new study set out to examine this genetic connection more closely, using mice with the same immune alteration.
While some of these mice spontaneously developed inflammation in their intestinal tracts as well, the level of severity varied and appeared to be made worse by the presence of certain bacteria and a low fiber diet. In fact, mice bred to lack any bacteria had no evidence of disease.
By adjusting the presence or absence of a model human gut microbiome and the dietary fiber content for their mouse models, the team found they could turn up or down inflammation. What’s more is the inflammation triggered by fiber free diets appeared to increase in reaction to the increased abundance of mucin degrading bacteria Akkermansia mucinphila and Bacteroides caccae.
“These bacteria start foraging on the mucus layer for nutrients, reducing its thickness and barrier function and brings microbes just 10-100 microns closer to the host tissue. That was enough in the context of the mice with IBD genetics to make them sick,” said the senior author.
On the other hand, feeding the mice a fiber rich diet prevented inflammation from developing and even returning mice fed low fiber to a high fiber diet led to a peak in inflammation followed by a decline, suggesting fiber can reverse the deleterious effects of mucus erosion on inflammation. But ironically, IBD, especially in children, is often treated with a formula-based diet called exclusive enteral nutrition (EEN), which lacks fiber. Despite a lack of fiber, this diet does result in reduced inflammation in people, for reasons unexplained.
To answer why, the team dried down the formula to administer it to the mice with IBD genetics. Interestingly, these mice had varying levels of inflammation following the diet, with some experiencing considerably less inflammation than the mice fed the fiber free diet, even though the EEN diet also reduced mucus thickness.
The team then discovered that elevated amounts of a single branched-chain fatty acid, isobutyrate, was elevated in EEN fed mice and might suppress inflammation. Isobutyrate is produced through fermentation by some bacteria in the gut. “It seems one of the ways these exclusive enteral nutrition diets could possibly work in people is by triggering certain bacteria to make beneficial metabolites,” said the author.
Spurred by these findings, the team plans to further interrogate how diet and bacteria interact to improve on therapies for pediatric IBD as well as answer how to potentially reverse or prevent the onset of these diseases by manipulation of these environmental triggers.
"Our findings suggest a potential new path for treating IBD. By tailoring specific dietary interventions to influence gut microbiome function, we may be able to manipulate these bacterial communities to alleviate inflammation.” said the author.