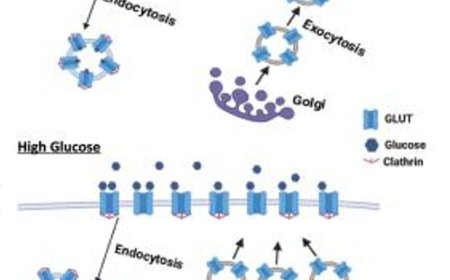
How weight loss drugs target the brain and pancreas
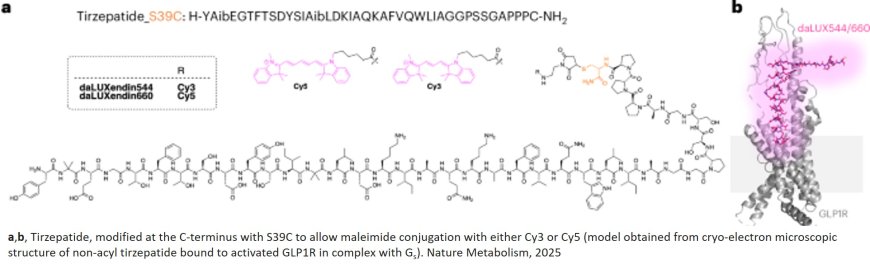
An international research team have developed a novel imaging approach to track how popular dual agonist drugs like tirzepatide interact with cells in the pancreas and brain. Published this week in Nature Metabolism, the findings could support the design of more effective treatments for diabetes and obesity.
The team created fluorescently tagged versions of dual-acting molecules that mimic the effects of tirzepatide - a drug that activates two key hormone receptors, GLP-1R and GIPR, to control blood sugar and appetite. These new chemical tools, called daLUXendins, allow researchers to visualise for the first time how such drugs move through the body and bind to specific cell types.
Tirzepatide (marketed as Mounjaro or Zepbound) is one of a new generation of therapies known as dual agonists, which stimulate both the glucagon-like peptide-1 receptor (GLP-1R) and the glucose-dependent insulinotropic polypeptide receptor (GIPR). These receptors are found not only in the pancreas, where they help regulate insulin release, but also in the brain, where they influence appetite.
Until now, the exact cellular targets of dual agonist drugs remained unclear. Antibodies used for receptor detection can be unreliable or unavailable, limiting researchers’ ability to trace drug pathways in tissue.
“By using our new dual fluorescent probes, we can now track the receptors simultaneously in live tissues and identify which cells respond to treatment,” said the study co-author. “This provides a powerful tool for understanding how dual agonists achieve their metabolic effects.”
“We are particularly interested in the dynamics of the peptides,” said study lead author. Using daLUXendins and super-resolution microscopy, the team showed that these fluorescent probes bind strongly to beta cells in the pancreas, and also to alpha and delta cells, offering insight into the range of metabolic effects triggered by tirzepatide.
In the brain, the researchers were able to visualise the drug reaching regions involved in appetite regulation, including specialist cells known as tanycytes that monitor nutrient signals and communicate with feeding centres.
The study also found that the receptors form tiny clusters or ‘nanodomains’ in the pancreas, which may help explain how dual agonists amplify signals, for instance through cumulative effects.
While the research relied on mouse models and fluorescent surrogates of tirzepatide, the authors believe the approach can be adapted to human studies and expanded to include other targets, including new triple agonists that also act on glucagon receptors.
The study provides important insights into why dual agonists are so successful. At the same time, it raises new questions: What would happen if the drugs' access to the brain were improved? And how do new triple agonists with additional glucagon content work?
The author added: “The next step will be to see how these results differ from triple agonists, which are more effective again."
https://www.nature.com/articles/s42255-025-01342-6
https://sciencemission.com/Fluorescent-GLP1RGIPR-dual-agonist