GUK1 boosts lung cancer metabolism to fuel growth
Lung cancer is a particularly challenging form of cancer. It often strikes unexpectedly and aggressively with little warning, and it can shapeshift in unpredictable ways to evade treatment.
While researchers have gleaned important insights into the basic biology of lung cancer, some of the disease’s molecular maneuvers have remained elusive.
Now, a team of scientists has made strides in understanding how a genetic flaw in some lung cancers alters cancer cell metabolism to fuel the disease.
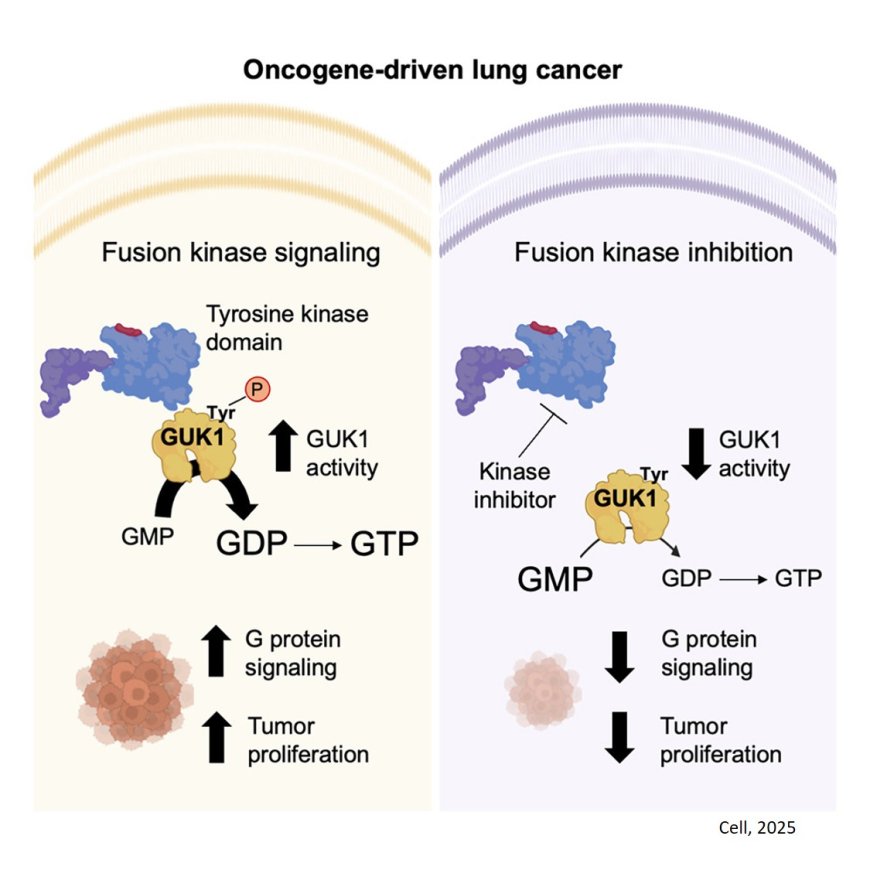
Working with mouse models and human cancer cells, the researchers identified a metabolic enzyme called GUK1 in lung cancers harboring an alteration in the ALK gene. Their experiments showed that GUK1 plays an important role in boosting metabolism in tumor cells to help them grow.
The findings, reported in Cell and supported in part by federal funding, provide a clearer picture of how metabolism works in lung cancer.
The research could set the stage for developing therapies that target GUK1 to curb cancer growth, the team said.
“A huge percentage of patients I see in the clinic do well for some period of time on the currently available therapies, but eventually relapse,” said co-first author.
Cancer cells must change their metabolism in order to continue growing and surviving amid attacks mounted by the immune system and cancer treatments, the senior author explained.
“Our goal was to understand how specific cancer gene aberrations might directly rewire metabolic pathways to enable cancer growth,” the author said.
The author deems cancer metabolism an emerging area in cancer research, and one that could inform the design of a new generation of precision cancer therapies targeted directly at the cellular processes that ignite tumor growth.
The researchers set out to study lung cancers caused by an alteration in the ALK gene that leads to the production of an abnormal ALK protein. First, they screened the metabolic proteins present in these ALK-positive cancers, and identified GUK1 as one of particular interest.
“We were really intrigued by what the interaction between ALK and GUK1 means — and like metabolic detectives, that’s what we followed,” the author said.
Next, they conducted a series of experiments in mice and patient-derived cancer cells to explore GUK1’s contribution to metabolic changes in ALK-positive cancer cells.
The scientists determined that GUK1 is an enzyme that helps abnormal ALK proteins make a molecule called GDP, a precursor to the energy-rich molecule GTP that cancer cells need for tasks such as dividing and making proteins. When the researchers disabled GUK1, cancer cell growth slowed considerably, suggesting that ALK-positive cancers become highly dependent on this enzyme as their molecular fuel for mischief.
“GUK1 turned out to be a metabolic liability in this subset of lung cancer that facilitates tumor growth and survival,” the co-lead said.
The team also found evidence of elevated GUK1 levels in additional subtypes of lung cancer, suggesting that the enzyme may play a role in lung cancers driven by other genetic defects.
“By focusing on the basic biology of lung cancer, we were able to identify a new metabolic mechanism that is important in the disease,” the senior author said.
The researchers note there is a lot more to be uncovered about GUK1 in cancer. They are interested in exploring how many types of cancer are driven by GUK1 in some way. They also want to understand in greater detail how inhibiting GUK1 affects cancer cells. Finally, given that many patients with lung cancer eventually relapse, they want to study whether and how GUK1 helps cancer cells metabolically reprogram themselves to sidestep treatment.
If GUK1 is indeed a key enzyme that gives various cancers the metabolic boost they need to grow quickly and persistently, it could be a compelling target for new cancer therapies.
“We hope that identifying distinct metabolic vulnerabilities like GUK1 will open up new avenues for therapeutic targeting in cancer patients in the future,” the co-lead said.
https://www.cell.com/cell/abstract/S0092-8674(25)00093-5