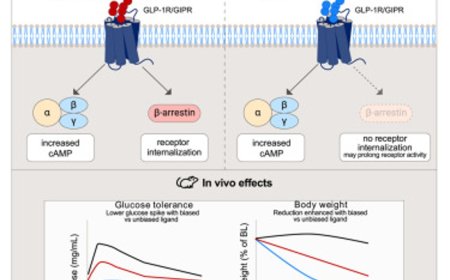
Immune-related colitis treatment after cancer immunotherapy
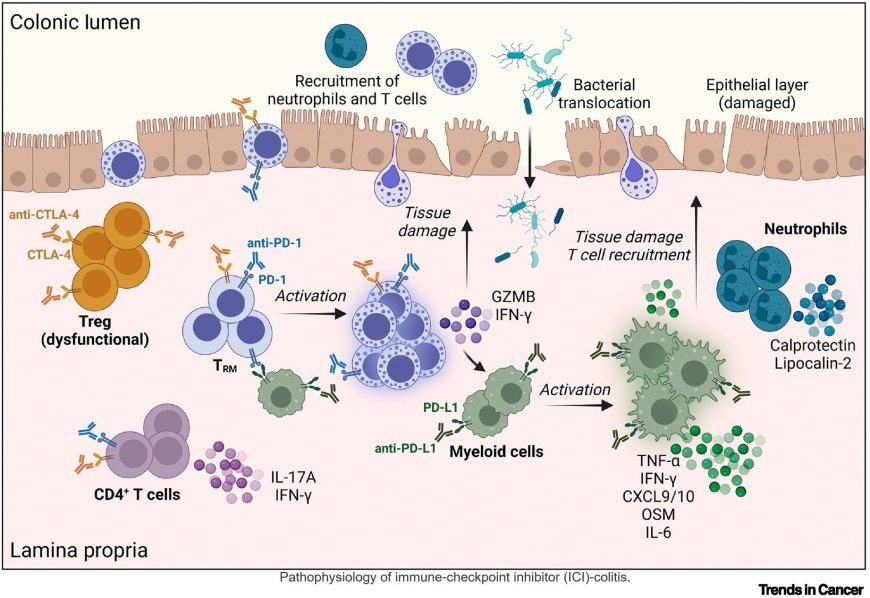
A significant fraction of patients treated with immune-checkpoint inhibitors (ICIs) develop immune-related adverse events (irAEs).
ICI-colitis can be fatal if not treated in a timely manne r. ICI-mediated colitis is driven by the activation of intestinal CD8+ tissue-resident memory T cells (TRM cells), activated T helper 1 cells (Th1 cells), and proinflammatory myeloid cells.
Biomarkers for ICI-colitis diagnosis and therapy response are needed and may include inflammatory cytokines and fecal calprotectin.
Immunosuppressive therapies are limited for patients refractory to corticosteroids and second-line immunosuppression.
Novel immunomodulatory therapies should resolve inflammation in the target organs, without comprising antitumor immunity. Fecal microbiome transplantation (FMT) or extracorporeal photopheresis (ECP) may effectively target ICI-colitis, while not affecting antitumor immunity.
https://www.cell.com/trends/cancer/fulltext/S2405-8033(25)00174-8