New bacterium that causes gut immunodeficiency
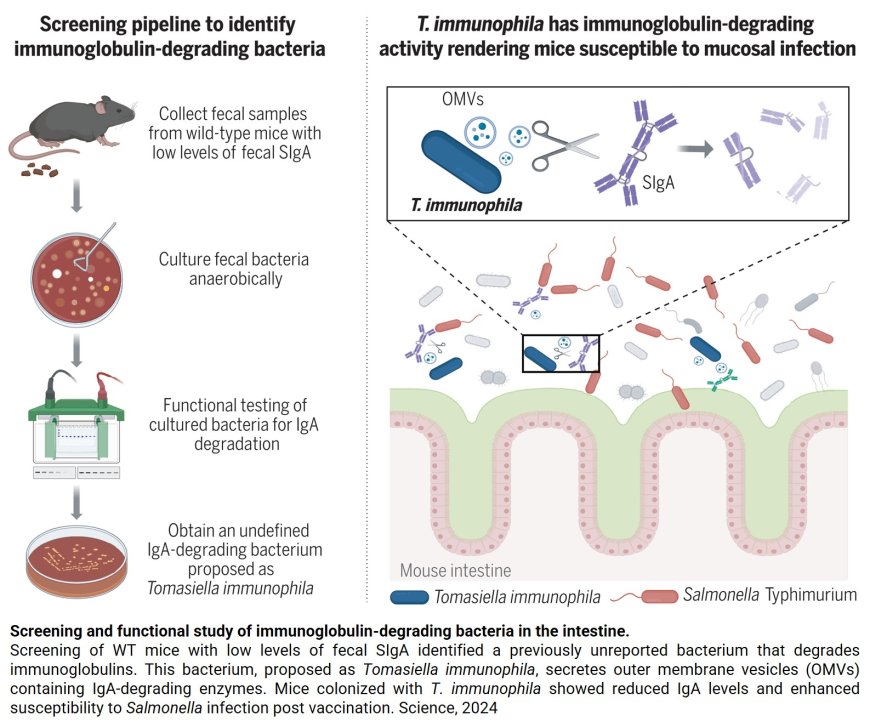
Researchers have discovered a new bacterium that weakens the immune system in the gut, potentially contributing to certain inflammatory and infectious gut diseases.
The team identified the bacterium, Tomasiella immunophila (T. immunophila), which plays a key role in breaking down a crucial immune component of the gut’s multi-faceted protective immune barrier.
Identifying this bacterium is the first step to developing new treatments for a variety of inflammatory and infectious gut diseases. These conditions, including inflammatory bowel disease, Crohn’s and ulcerative colitis, are associated with decreased levels of secretory immunoglobulin A (SIgA), an antibody that protects mucosal surfaces.
The study was published in Science.
“Our research represents a critical role of a specific component of the gut microbiome in human health and disease,” said the senior authopr. “By identifying this specific bacterium, we have not only enhanced our understanding of gut diseases but also opened a promising new avenue for treatment. Pinpointing the culprit behind the breakdown of the gut’s protective adaptive immune barrier is a significant step toward developing much-needed therapies for conditions like inflammatory bowel disease, Crohn’s and ulcerative colitis.”
In the gut, SIgA binds continuously to microbes, preventing them from reaching and damaging the body’s tissue. In previous research, the team discovered that intestinal bacteria could reduce SIgA levels, which can lead to increased risk of infection and excess inflammation.
In this new study, researchers found that T. immunophila’s presence in the gut increases susceptibility to pathogens and delays repair of the gut’s protective barrier. T. immunophila’s name is an homage to a pioneer in immunology. SIgA was discovered by Dr. Thomas Tomasi, who published his findings in a foundational paper in Science in 1963.
The researchers found that T. immunophila was auxotrophic for N-acetylmuramic acid (MurNAc), a critical component of bacterial cell walls, which was essential for its optimal growth in vitro.
T. immunophila alone did not colonize WT conventionally raised or germ-free mice. However, fecal slurries from IgA-high mice facilitated T. immunophila colonization in WT mice, suggesting that helper strains are crucial for its successful colonization in the mouse intestine. As a result, mice colonized with T. immunophila exhibited reduced levels of SIgA in the intestine and increased susceptibility to mucosal pathogens such as Salmonella Typhimurium and Candida albicans.
Furthermore, these mice also showed delayed mucosal barrier repair in response to dextran sulfate sodium-induced intestinal injury. Additionally, mucosal exposure to T. immunophila in mice induced the production of intestinal SIgA specific to this bacterium. T. immunophila secreted multiple types of immunoglobulin-degrading proteases associated with its outer membrane vesicles and these proteases were observed to specifically degrade all isotypes and subclasses of mouse antibodies; the enzymatic activity did not extend to unrelated proteins.
The degradation of immunoglobulins by T. immunophila was particularly selective for rodents. Recombinant antibodies that contain the mouse kappa chain were cleaved by T. immunophila regardless of the species of the heavy chain. Notably, T. immunophila preferentially degraded antibodies harboring kappa light chains while sparing those with lambda light chains.
These findings emphasize the important role of symbiotic bacteria such as T. immunophila in mucosal immunodeficiency, providing potential insights into related human diseases.
https://www.science.org/doi/10.1126/science.adk2536
https://sciencemission.com/A-host-adapted-auxotrophic-gut-symbiont-induces-mucosal-immunodeficiency