Stem cells tightly regulate dead cell clearance to maintain tissue fitness
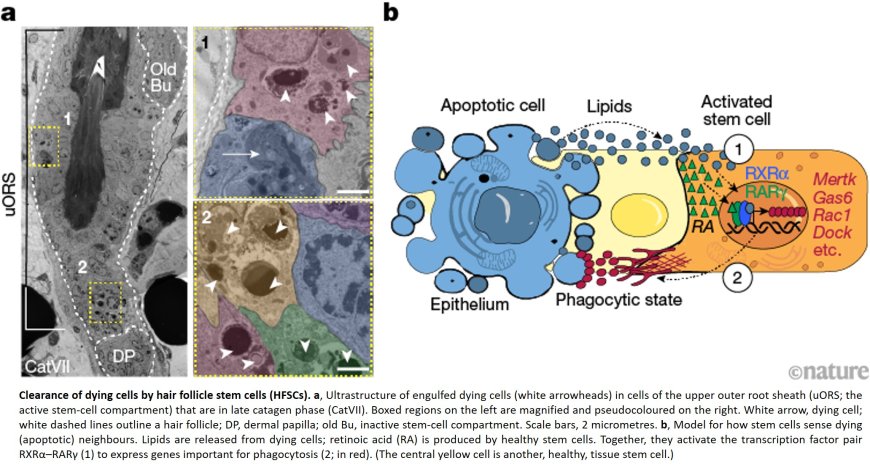
Billions of our cells die every day to make way for the growth of new ones. Most of these goners are cleaned up by phagocytes—mobile immune cells that migrate where needed to engulf problematic substances. But some dying or dead cells are consumed by their own neighbors, natural tissue cells with other primary jobs. How these cells sense the dying or dead around them has been largely unknown.
Now researchers have shown how the sensor system operates in hair follicles, which have a well-known cycle of birth, decay, and regeneration put into motion by hair follicle stem cells (HFSCs). In a new study published in Nature, they demonstrate that a duo of sensors works in tandem to pick up signals from both dying and living HFSCs, removing debris before tissue damage can occur and ceasing operation before healthy cells are consumed.
“The system is seemingly spatially tuned to the presence of corpses, and it only functions when each receptor picks up the signal is attuned to,” says the first author. “If one of them disappears, the mechanism stops operating. It’s a really beautiful way to keep the area clean without consuming healthy cells.”
“By diverting their attention towards eating their dying neighbors, HFSCs keep inflammation-generating immune cells away,” says the head of the lab. “They also likely benefit from these extra calories, but as soon as the debris is cleared, they must quickly return to their jobs of maintaining the stem cell pool and making the body’s hair.”
Every single hair follicle on your head goes through a specific cycle: growth, destruction, rest, and—as seen in your shower drain—shedding.
To initiate the process, hair follicle stem cells (HFSCs), located in the “bulge” of the follicle’s upper root sheath, signal to epithelial and mesenchymal cells, sparking growth. This stage takes its time, lasting from two to six years.
The destructive, or catagen, stage that follows is brief but intense, obliterating about 80% of the hair follicle in just a few weeks. The process begins at the follicle base and works its way upwards towards the HFSC niche. The result is a mass of dying and dead cells that need removal to prevent the resulting decay from triggering inflammatory or autoimmune responses.
Normally this would be the job of phagocytes like macrophages, but few are found in the hair follicle, meaning it must fall to local epithelial cells to keep things tidy. Stewart wanted to determine the chemical communication that manages the process.
The authors took a closer look at the catagen phase in mouse hair follicles, whose hair cycle is short and synchronous across the hair coat. It’s only at the later stage of catagen that the death signals originating in the follicle base finally reach the spot where undifferentiated stem cells reside. It had long been thought that stem cells were spared from the destruction, but surprisingly, the team found that some do die—and are engulfed by their neighbors.
They discovered that the clearing out can only begin when two receptors are both activated in the healthy cells. The first, called RXRα, detects the presence of lipids, one of several well-known “find me” signals secreted by a dying cell. The second, called RARγ, senses growth-promoting retinoic acid secreted by healthy cells.
Neither can activate the cleanup process alone. “A dying cell triggers the mechanism to begin, and when there are no dead cells left, the lipid signal disappears, leaving only the retinoic acid signal from the healthy cells,” the author says. “This tells the program to dampen back down. It’s so elegant in its simplicity.”
They also documented that macrophages were slow to migrate to the region, showing up as much as four days after cell death. “It’s been pretty broadly thought that professional phagocytes eventually swoop in and do the heavy cleanup, and that non-motile cells were a sort of backup system,” the author says. “I was very surprised to find that the hair follicle stem cells were actually the first responders, especially because mouse skin is fairly well-endowed with macrophages, so they’re not even that far away.”
They also found that tissue damage occurred when the HFSCs were prevented from clearing dying cells and left the work to the macrophages. This raises the possibility that genetic defects in this process might contribute to human skin pathologies, including inflammation and hair loss.
The HFSCs that consumed nearby dying cells—some ate as many as six of their neighbors—may benefit from ingesting the cells’ proteins, nucleic acids, solutes, and lipids. If that’s the case, the perks remain to be understood—a subject the Fuchs lab will investigate in the future.
“It’s possible that they can use that material to fuel their own growth or benefit from it in some other way,” the author says, “but it’s equally possible that it has negative effects. Maybe they’re too occupied with digesting all this material to take care of their normal duties.”
The findings have applications beyond the hair follicle, because it is only one of several areas of the body where there are few professional phagocytes around. In regions of the brain, breasts, and lungs, for example, epithelial and mesenchymal tissue cells, including stem cells, moonlight as ersatz phagocytes.
“We often use the phrase ‘you are what you eat,’” adds the senior author. “For our body’s stem cells, this may be their way of keeping tissues fit by clearing out naturally dying cells and guarding against inflammation.”