Autoantibody patterns in patients with rheumatoid arthritis
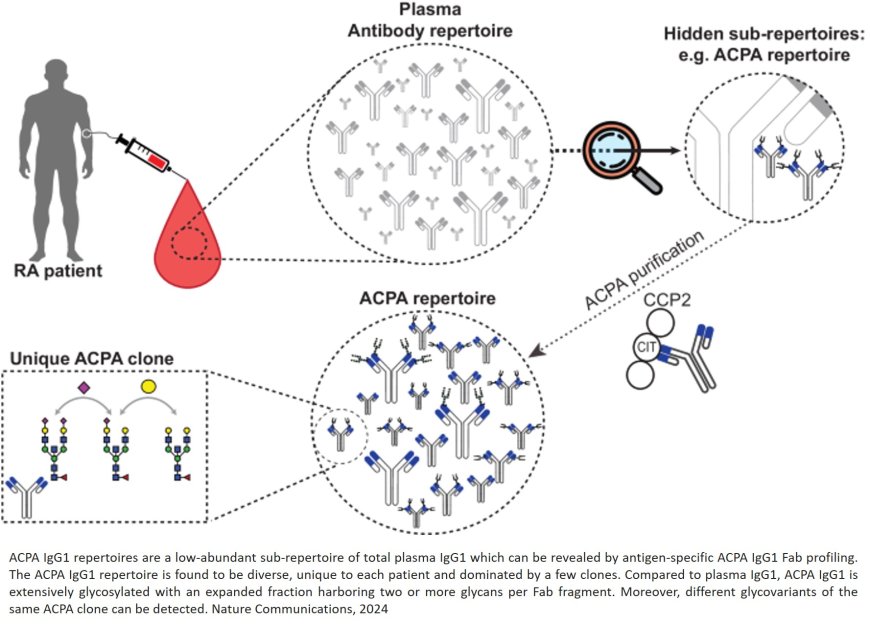
Patients with rheumatoid arthritis (RA) all have a unique and diverse set of antibodies that are involved in the development of the disease. Researchers unveiled the complexity of these antibodies using powerful lab tools capable of analysing our immune system at molecular levels. Their discovery suggests that current assumptions about the origin of RA are too simple. Still, their findings may point towards improved diagnostics.
Rheumatoid arthritis is a chronic autoimmune disease that primarily affects the joints, causing pain, stiffness, and swelling. It arises when the immune system mistakenly attacks the body’s own tissues, leading to inflammation in the joints and potentially other organs.
The exact cause of RA remains unknown, but a crucial role is played by antibodies, special proteins made by the immune system to help fight off infections. They recognize and attack specific targets, like viruses or bacteria. Some antibodies are wrongly produced, causing them to attack our own body. Normally, our body’s immune system is equipped with a ‘filter’ that cleans up these so-called autoantibodies. Researchers believe that this mechanism is malfunctioning in RA patients.
The extent to which this filter is malfunctioning, now appears to be much greater than expected. Research published in Nature Communications, reveals that it’s not just a handful of different RA-associated autoantibodies that evade the filter. On the contrary, the researchers found an extremely broad variety of these antibodies.
The team used novel mass spectrometry tools that profile specific antibodies typically seen in the blood of RA patients, which are called anti-citrullinated protein antibodies (ACPAs). They discovered that each RA patient possesses a unique and diverse set of ACPAs. Their findings challenge previous assumptions about the backgrounds of RA, that overlooked the antibodies’ diversity and complexity. “This shows that RA is not just a disease occurring due to small errors, but a big structural problem in the immune system”, says the author.
The study also revealed that these ACPAs are extensively modified with sugar molecules, known as Fab glycans. Intriguingly, some antibodies had multiple sugar molecules attached. This is much more then researchers normally observe in antibody profiles.
Having extra glycans aboard, may help the ACPA antibodies pass the filter of the immune system, says the author. The immune system uses several very strict checks during antibody production, to make sure all antibodies are correct. Wrongly produced antibodies are then detected and removed. The author suspects that glycans could help ACPAs trick the control system, allowing ACPAs to pass through the filter and form the onset of RA.
Current efforts to develop treatments for RA are mainly geared towards eliminating autoantibodies directly. This strategy may not be effective, says the author. “When you realize that there is such an extreme diversity in RA-related autoantibodies, it seems virtually impossible to eliminate them. A better approach may be to intervene earlier in the disease process, by targeting the malfunctioning filtering mechanism that allows autoantibodies to pass through.”
Understanding these unique proteins is important, as it could ultimately also help doctors diagnose RA better. “When more molecular details about RA-related antibodies are uncovered, the disease may be diagnosed in an earlier stage”, says the author. “Even though RA remains an incurable disease, with an earlier diagnosis you can take better measures to control its progression.”